CAMBRIDGE, Mass., June 8 -- Amyloid fibers are clumps of plaque-like proteins that clog up the brains of people with neurodegenerative illnesses such as Alzheimer's diseases and of Creutzfeldt-Jacob disease, the human analog of mad cow disease. But even though amyloids are common and implicated in a host of conditions, researchers haven't been able to identify their precise molecular structures. Conventional techniques used to capture images of proteins, such as x-ray crystallography and nuclear magnetic resonance imaging, don't work with fibrous structures such as amyloids, and scientists depend on high-resolution images of molecules to study their function.
Now researchers have found a way to work around these limitations, illuminating the configuration of these sometimes pernicious molecules. The results provide hints of why mad-cow type diseases tend to have a difficult time jumping species.
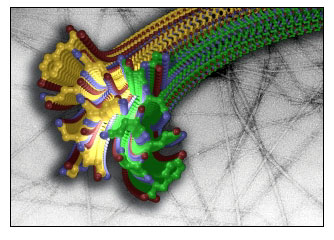
This structure, determined by x-ray microcrystallography, is the first glimpse at the atomic level of the amyloid spine that is common to fibrils formed from numerous proteins, some associated with fatal diseases. The "molecular zipper" is highlighted. (Credit: Micahel Sawaya, Rebecca Nelson, Melinda Balbirnie and David Eisenberg, University of California, Los Angeles)
"These findings give us some fundamental insights in how amyloid fibers form," said WSusan Lindquist, lead scientist in the research team at the Whitehead Institute for Biomedical Research, in Cambridge, Mass. "They solve the important problem of identifying the intermolecular contacts that hold the amyloid fiber together."
Amyloid fibers are often composed of prions -- proteins that misfold and recruit neighboring proteins to misfold as well, a process that Lindquist calls a "conformational cascade." When such a cascade occurs, the prions join and form amyloid fibers. (While not all amyloids are composed of prions, all known prions, in their transmissible states, form amyloid fibers.) Still, many scientists have been frustrated by their inability to gain anything more than a limited understanding of an amyloid's architecture.
Rajaraman Krishnan, a postdoctoral researcher in Lindquist's lab, found a way around that problem using strains of yeast. Rather than develop a single high-tech method for solving the amyloid structure, he used a combination of low-resolution tools to analyze varieties of prion strains and piece together the puzzle of how amyloids form.
"We now have an overall picture of how prions join together to form the amyloid's molecular structure," said Lindquist, who also is a professor of biology at the Massachusetts Institute of Technology (MIT), which is affiliated with the Whitehead Institute.
Prions are in the business of converting other prion molecules to join their ranks. As they join together, they can create an amyloid fiber. To understand the nature of this fiber, it's necessary to understand how the prions that comprise it attach to each other. Krishnan was able to identify the precise segment at which the prions interact -- something no one had done before with a real prion.
Krishnan took a variety of yeast prion strains and modified them so that if designated regions came into contact with each other, they would emit a fluorescent signal, allowing him to map the pattern by which the different strains of prions interacted with each other.
He found that each prion molecule had only two points at which they connected to other prion molecules. One point he called the "head," the other the "tail." The head of one prion would only interact with the head of another prion, and likewise with tails. Remarkably, the same prion from the same yeast species could sometimes fold differently, and this fold would form its own cascade of interactions. In this altered form, the prion molecules interact in slightly different places, presenting different surfaces to promote the conversion of other prion molecules.
Lindquist said the techniques used in this study will ultimately prove useful for studying prion strains found in mammals like mice, cows and, ultimately, humans.
"This gives us insight as to why some prions can't cross the species barrier, while others can -- as they have with mad cows and humans," said Lindquist.
That gap has also been observed between other species, she said. "In fact, some type of prions from infected hamsters can't make the species jump into mice, while others do and vice versa."
While the results of this research are clearly of interest to scientists investigating conditions such as Alzheimer's, it's also relevant to scientists studying nanotechnology. In March 2003, Lindquist published a paper in the journal Proceedings of the National Academy of Sciences in which she described how amyloid fibers can become the core of nanoscale electrical wires, opening the possibility of one day incorporating them into integrated circuits.
"These findings are quite relevant for the material sciences," said Lindquist. "The more we understand about how these fibers work, the more we can get them to self-assemble" -- a key advantage for nanoscale devices that are very difficult to manipulate directly. In addition, amyloids are unusually robust, which makes them attractive for nanodevices. The advantage of the yeast protein is that it is nontoxic, even for yeast.
The research, to be published this week in the journal Nature, was supported by the DuPont-MIT Alliance and an NIH grant.
For more information, visit: www.whitehead.mit.edu