Hey, the next time you have a party, don’t forget the ripe bananas!
That’s one conclusion you might come to after reading a study on the breakdown, or catabolization, of chlorophyll – the process that takes place in autumn leaves as they turn from green into reds, yellows and browns. After discovering recently that the ripening process of apples and pears is chemically the same as the degreening of leaves, scientists from the University of Innsbruck in Austria and from Columbia University in New York City, led by Dr. Bernhard Kräutler, fortuitously added a new fruit to their study – bananas.
 The investigators discovered that the catabolites of bananas are colourless, as they are in apples and pears, and that, similarly, many are nonfluorescing. However – the surprise – certain ones, fluorescent chlorophyll catabolites, or FCCs, luminesce under ultraviolet light – known to partygoers as black light – making bananas appear bright blue at 450- to 470-nm wavelengths. About 10 years earlier, FCCs had been observed to exist fleetingly in degreened leaves of some higher plants, but their discovery in fruit is brand-new. The results were reported in the 3 November 2008 issue of Angewandte Chemie.
The investigators discovered that the catabolites of bananas are colourless, as they are in apples and pears, and that, similarly, many are nonfluorescing. However – the surprise – certain ones, fluorescent chlorophyll catabolites, or FCCs, luminesce under ultraviolet light – known to partygoers as black light – making bananas appear bright blue at 450- to 470-nm wavelengths. About 10 years earlier, FCCs had been observed to exist fleetingly in degreened leaves of some higher plants, but their discovery in fruit is brand-new. The results were reported in the 3 November 2008 issue of Angewandte Chemie.
Unripe, or green, bananas are barely visible under UV light because their peel contains only small amounts of FCCs. However, as a banana ripens to a bright yellow, its FCCs accumulate significantly in the peel, peaking in number when the banana is ripest. When ready to eat, it glows a deep medium-blue under UV light. It turns out that the FCCs are optical brighteners, too, so that, even in the visible spectrum, bananas seem to glow a little brighter.
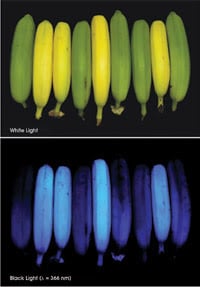
Ripe bananas appear yellow under white light (above); under ultraviolet light, they luminesce blue (below).
Besides fluorescing catabolites, the other surprise about bananas is a propionate ester, a functional group that had never been identified in a chlorophyll breakdown product. The investigators discovered the propionate ester as a chemical modification of FCCs, which slows down their inevitable transformation into nonfluorescing breakdown products.
In bananas, FCCs accumulate and disappear slowly – over a matter of days – whereas in higher plants, they are short-lived intermediate breakdown products, disappearing at the rate of their transport process from the chloroplast into the vacuole. Once the FCCs are in the vacuole, they disappear within half an hour.
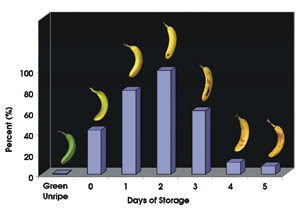
This bar graph shows the relative amount of fluorescent chlorophyll catabolites (FCCs) in a ripening banana peel. The chemical breakdown process is identical to that of leaves as they turn colours in autumn. The percentage of FCCs peaked when the banana was most ripe – but before it was overripe. Photos courtesy of Bernhard Kräutler. ©Angewandte Chemie.
Because not all animals see in the same spectral range, a fruit’s colour determines which species can see it. To the birds, bees and insects that can see ultraviolet light, that ripe banana will look blue. But in case you’re wondering – primates such as monkeys see only in the visible spectrum, so if there are any such animals at your party, you still will need that black party light to entertain them with blue bananas.