Longer wavelengths open the way to deeper studies of brain processes, with inherent 3D resolution. But challenges remain before this technique can be fully exploited.
DARRYL MCCOY AND MARCO ARRIGONI, COHERENT INC.
One of the most active and exciting areas in neuroscience is the use of optogenetics to unravel how neural pathways in the brain process and transmit information. Optogenetics involves the use of light-sensitive opsins — a special class of membrane-embedded proteins. Opsins are switchable channels or pumps for specific cations or anions with on or off states determined by irradiation with light of an appropriate wavelength. It has been known for decades that neurons transmit signals in the form of an action potential or voltage spike along their membranes. If opsins are present in a neuron’s membrane, light can be used to influence the flow of charged ions across the membrane, mimic or inhibit the initiation of an action potential, and hence turn neuron activity on and off.
Small lab mammals such as mice can be genetically modified so that specific opsins are produced in their neurons, or even selectively in only certain types of neurons, enabling optogenetic studies of live animals. Specifically, the noninvasive, noncontact nature of light enables optogenetics to be conducted in the cortex and neocortex of these live animals via a small glass window that replaces the upper part of the cranium. The shift to longer laser wavelengths means that some studies can even be conducted directly through the cranium, albeit usually requiring prior thinning of the skull.
Figure 1. Courtesy of Lloyd Russell, Hausser Laboratory, University College London.
In “all-optical” studies, sometimes called all-optical physiology, light is used to manipulate neurons using opsins and to measure activity in the local network (Figure 1). That is possible because neural activity usually triggers increased calcium ion Ca2+ concentrations, which can be readily imaged using fluorescent calcium indicators. The burgeoning range of genetically expressed Ca2+ probes provides a powerful tool for this purpose. Since Ca2+ concentration and neural activity do not correlate 100 percent of the time, and changes in Ca2+ are slower than the actual voltage changes, there is also growing interest in genetically expressed probes with fluorescence that is modulated directly by membrane voltage.
Femtosecond lasers preferred for multiphoton excitation
Several types of light sources, including filtered lamps and LEDs, can be used to stimulate or excite neurons. However, lasers provide unique benefits of spatial selectivity and high brightness; femtosecond lasers in particular offer specific advantages thanks to their capability to excite multiphoton processes. When tightly focused in a live or fixed-tissue sample, the femtosecond laser can drive two- (or even three-) photon excitation of opsins and metabolic probes with minimal photodamage, and with inherent three-dimensional resolution. (Multiphoton excitation has a highly nonlinear dependence on light intensity and only occurs at the beam waist of the focused laser.) This spatial resolution enables activation and interrogation with single neuron resolution.
Optogenetics is still in its infancy. Many important scientific discoveries using this technique lay ahead. There also are several challenges yet to be overcome before this powerful scientific tool can be fully exploited.
Minimizing crosstalk
A key challenge in all-optical studies is to minimize crosstalk — ensuring that the laser light used for photoactivation does not significantly excite fluorescent signals from the interrogation probes, or vice versa.
One approach to this problem is to use photoactivators and probes with absorption peaks that are well-separated (Figure 2). Here, two ultrafast laser wavelengths are focused into the sample. Wavelength 1 is used to activate neurons by two-photon excitation, and wavelength 2 is used to image activity in other neurons, again via two-photon excitation.
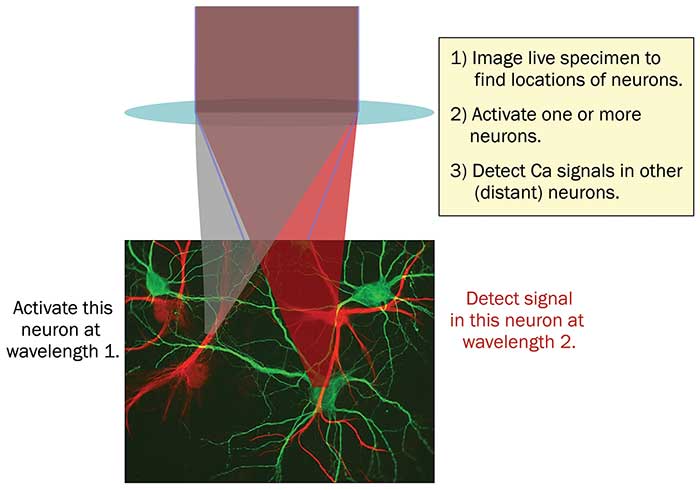
Figure 2. In all-optical physiology experiments with multiphoton lasers, neural activity can be manipulated and monitored at single neuron resolution. The use of two separate wavelengths for photoactivation and detection enables minimization of crosstalk between these ‘write & read’ channels. Courtesy of Coherent Inc.
In addition to requiring two independent laser wavelengths, this setup requires the availability of photoactivators and calcium probes with well-separated spectra. Unfortunately, the two-photon spectra of most activators and probes are quite broad — as much as 50 to 100 nm, or even more — and generally wider than the corresponding one-photon spectra. However, developments in this area have been quite rapid. For example, several red-shifted photoactivators have recently been added to the all-optical toolkit, including C1V1, ReaChR and Chrimson. The one-photon absorption peaks can be as long as 600 nm. In theory, these can be used with a range of blue excited Ca2+ probes. Nevertheless, the absorption spectra of most activators have a weak, but not negligible, short wavelength tail and complete separation with this scheme has proved challenging. Conversely, researchers can use one of the new red-shifted calcium indicators, such as the RCaMP family, which have one-photon absorption peaks around 550 nm, together with a shorter wavelength photoactivator, such as ChR2, with a one-photon absorption peak closer to 460 nm. Alas, even this is not proving a straightforward route to eliminating crosstalk.
Peak intensity for photoactivation
Initial, proof-of-principle, all-optical experiments involved just a handful of neurons. However, the mammalian brain comprises massive interconnections between myriad neurons. To begin to unravel the mysteries of the brain, researchers want to simultaneously study larger populations of neurons. Current state-of-the-art experiments involve a few tens of neurons, but some neuroscientists are already discussing the need to study at least 1000 neurons, which would typically encompass a column of tissue 250-µm across with a depth up to 1 mm.
The main challenge is the peak intensity needed for photoactivation. That is partly because numerous channels have to be recruited to reach a threshold voltage effect. This challenge is compounded by the lower activation cross section intrinsic in two-photon excitation. One frequently used solution to address this challenge was developed by David Tank at Princeton University. His method consists of scanning the focused laser beam in a spiral pattern, progressively covering the entire neuron body or soma until the action potential is produced. This may take something like 30 ms for each neuron. Additionally, or as an alternative approach, the laser power can be divided to cover selective areas of several neurons using computer-controlled holographic illumination with a spatial light modulator (SLM), as developed by Valentina Emiliani at Université Paris Descartes.
Whether the excitation is truly simultaneous or fast but sequential, photoactivation of larger neuron populations inevitably requires higher laser powers — at least several watts of output at the laser, and maybe even tens of watts, although delivered over milliseconds only. This demand for higher power is somewhat offset by the need to avoid photodamage and tissue heating, both of which can be ameliorated by using a low-duty cycle and spreading the power among multiple spatially separated targets.
Ytterbium fiber: higher power at longer wavelengths
All-optical studies have created a need for femtosecond lasers emitting multiple watts in the 1040- to 1150-nm range. These wavelengths are at or beyond the limit of the Titanium:Sapphire (Ti:S) lasers that have been the workhorses of multiphoton microscopy for the past 15 years. Further, while a Ti:S-pumped optical parametric oscillator can reach this wavelength range, the power may be inadequate for studying large neuron populations.
Ytterbium-doped crystals are alternative gain materials, but power scaling causes problems because of cooling and thermal lensing of the bulk gain material. In response, laser manufacturers have developed a new type of femtosecond laser based on ytterbium-doped fiber that can be scaled to several watts, or even tens of watts, at 1030 to 1070 nm. While the first Yb-doped fiber lasers were unable to produce multiwatt powers with pulse durations shorter than about 300 fs, engineers at Coherent found solutions to balance nonlinearity and gain in these high-power lasers. These proprietary approaches have spawned several families of ytterbium fiber-based lasers that are well-suited to optogenetics as well as other near-IR applications. Some of these incorporate an integrated optical parametric oscillator that provides widely tunable output across the near-IR.
Industrial reliability and stability
Solving the pulsewidth/power trade-off in ytterbium fiber provides the combinations of peak and average powers required for all formats of optogenetic photoactivation. For example, the most compact of these new lasers delivers more than 2 watts of power at a fixed 1070 nm with a pulse-width <55 fs. By combining a more powerful ytterbium fiber with an optical parametric oscillator (OPO) in a single package, the Chameleon Discovery provides widely tunable output, as well as 1.5 watts at a fixed 1040 nm, enabling single-laser simplicity for these dual-
wavelength, all-optical experiments. Thanks to the power scaling potential of ytterbium fibers, other products now offer several watts of tunable output across the near-IR like Monaco with its Opera-F family of optical parametric amplifiers (OPAs).
Concepts from the world of industrial lasers, such as uptime and data throughput, are positively affecting the world of scientific lasers, minimizing the time from complex experiments to publication of results. The latest scientific lasers are now designed for high reliability 24/7 operation, using rigorous industrial protocols such as Highly Accelerated Laser Testing/Highly Accelerated Stress Screening (HALT/HASS). The accompanying stability is a particular advantage in so-called “chronic” imaging. Here all optical experiments on a target animal are repeated over a period of days or even months to monitor the effects of things like stress, task learning and effects of various drugs. It is therefore vital that the lasers deliver a repeatable performance over time.
Recent discoveries
Several research groups are already beginning to publish data from experiments based on these new ytterbium fiber lasers. For example, postdoctoral researcher Alan Mardinly and others in the Adesnik Lab at UC Berkeley have successfully used the Coherent Fidelity and Monaco lasers to activate a wide assortment of opsins, including ChR2, C1V1, Chronos and GtACR1. The high pulse energy of the Monaco and high average power of the Fidelity-HP have proved ideal for activating or suppressing large sets of neurons. Even more importantly, they can be effectively used with holographic wavefront shaping so that all the targeted neurons can be activated in nearly the exact same instant. This allows for precise temporal control over large populations of neurons in full three dimensions in the intact brains of behaving animals (Figure 3). This will open new possibilities of addressing how information is encoded in the nervous system in the precise timing of individual action potentials in large populations.
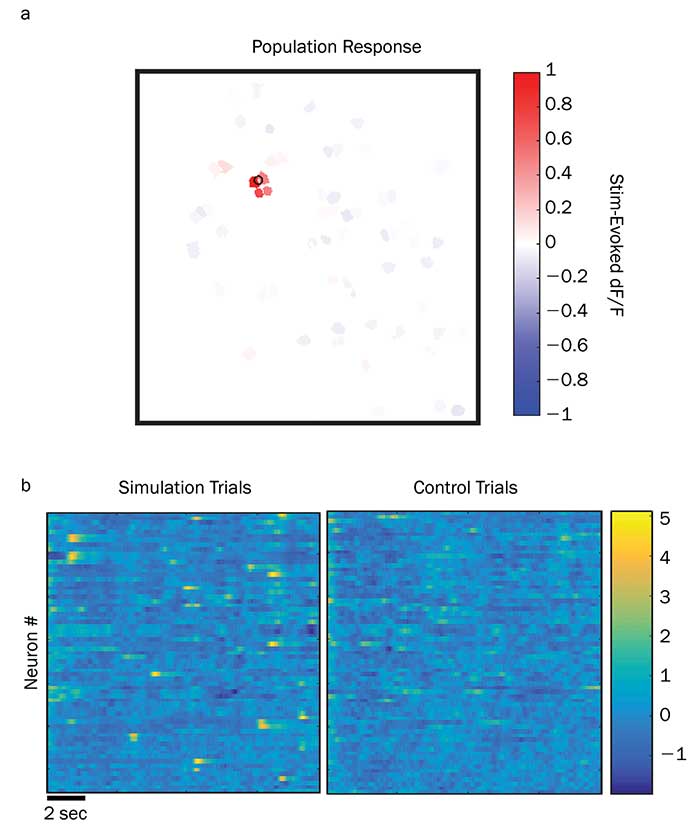
Figure 3. The results above come from a test study of holographic photostimulation pyramidal neurons in the live brain of a mouse during an active treadmill study. The upper panel shows how the programmable phase mask can be used to produce a single target spot, which in this case causes a calcium fluorescence change (dF/F) in four directly targeted neurons (a). The lower panel is a statistical plot showing normalized (z-score) fluorescence deviations using a time-varied phase mask (b). Here each data column is a time window of approximately 30-msec duration. Each row is a different specific neuron. This trial shows that this phase map method and multiwatt power at 1040 nm enables neurons to be targeted with high spatial selectivity and high temporal resolution. Courtesy of Alan Mardinly and Hillel Adesnik, UC Berkeley.
Professor Michael Hausser’s research group at University College London is on the forefront of development and utilization of all-optical experiments using two-photon excitation. They were one of the first to acquire a 2-watt ytterbium fiber laser and then a >18-watt laser.
Senior research associate Adam Packer explained a typical experiment. “We express a red-shifted photoactivator together with a shorter wavelength Ca2+ indicator in neocortical neurons in vivo. Crosstalk is minimized by using these long wavelength lasers — 1040 nm or greater — to excite the activator while imaging using the 920-nm output from a Ti:S laser.
“In our latest experiments, we have stimulated up to 50 neurons simultaneously at the same time as imaging the responses of these neurons and their neighbors. We image the neurons, select the targets and then create a pattern of focused spots using the SLM and the fiber laser,” Packer said.
Twin galvanometers in the beam path enabled Packer’s team to “jiggle” the beams in a spiral pattern.
“Using this strategy, we have found that we need 20 to 80 milliwatts of laser power at each neuron, for a duration of 10 ms for reliable neuron firing,” Packer said. “This power level appears to cause no significant photodamage or undesirable thermal perturbations. Moving to 20 watts at the laser enables firing more neurons with a good experimental overhead to enable all aspects of the activation and imaging to be fully optimized.”
Meet the authors
Darryl McCoy is a director of product marketing at Coherent Inc., managing the ultrafast tunable and fiber laser products for nonlinear microscopy and instrumentation markets; email: [email protected]. Marco Arrigoni is a director of strategic marketing at Coherent and covers the scientific research markets; email: [email protected].
Acknowledgments
The authors wish to acknowledge the valuable input and assistance of Adam Packer and professor Michael Hausser in preparing this document for publication.