The latest developments in lasers for microscopy are delivering a powerful combination of improved functionality and operational simplicity, and it’s benefiting both end users and OEMs.
DARRYL MCCOY, DANIEL CALLEN, MATTHIAS SCHULZE, AND MARCO ARRIGONI, COHERENT INC.
The optical microscope dates back to the time of Galileo. For most of the past century, microscopy was widely regarded as a ubiquitous and extremely mature field. Today, the picture is very different. Optical microscopy is an incredibly dynamic field with recent Nobel prizes given for new techniques that overcome the diffraction limit, as well as tools such as new fluorescent proteins that have enabled considerable advances in the life sciences.
![Figure 1. Deep imaging using simultaneous excitation at both 830 and 1100 nm with a Chameleon Discovery laser. The vessel wall of a mouse carotid artery showing en face and orthogonal views. The cyan structure shows collagen fibers (via second-harmonic generation [SHG] detection); the red are endothelial cell interfaces labeled with Alexa 647, and the green shows nuclei of the smooth muscle cells labeled with Cyto41. Courtesy of Dominic Depke, European Institute for Molecular Imaging (EIMI), Münster.](https://www.photonics.com/images/Web/Articles/2018/11/2/FEATMicroscopy_Fig1.jpg)
Figure 1. Deep imaging using simultaneous excitation at both 830 and 1100 nm with a Chameleon Discovery laser. The vessel wall of a mouse carotid artery showing en face and orthogonal views. The cyan structure shows collagen fibers (via second-harmonic generation [SHG] detection); the red are endothelial cell interfaces labeled with Alexa 647, and the green shows nuclei of the smooth muscle cells labeled with Cyto41. Courtesy of Dominic Depke, European Institute for Molecular Imaging (EIMI), Münster.
These dynamic changes all began with the introduction of the laser as a unique light source for microscopy. And although filtered lamps and LEDs have their uses as simple light sources in wide-field microscopy, the laser is at the heart of most of the advanced techniques and experiments involving fluorescence excitation.
Despite the sophistication of these techniques, most end users are not laser experts. Laser manufacturers are supporting a growing maturity with tools that provide an optimum combination of high functionality and operational simplicity.
Multiphoton microscopy
Multiphoton microscopy is unique in its ability to generate 3D images and 2D image slices deep within live tissue. Multiphoton imaging techniques are now widely used to study the live mouse brain cortex to help unravel the functioning of the mammalian brain. Here, researchers want to acquire deeper images at a faster rate to enable mapping of activity patterns in ever larger connected networks of neurons. This has driven increased functionality in ultrafast sources, such as a wider tuning range. It was a key factor behind the development of ytterbium fiber-based ultrafast lasers as an alternative to traditional titanium:sapphire (Ti:sapphire) lasers. An example is the Chameleon Discovery, which now provides a full octave-spanning tuning range. When it incorporates an optional frequency doubler, this laser provides gap-free wavelength coverage from 330 to 1320 nm (Figure 1).
Very recently, there have been equally important developments in ease of use for these new lasers, in the form of direct power modulation. Until now, a multiphoton microscope consisted of a tunable femtosecond laser source delivering a high-quality circular (TEM00) beam and a microscope that included the scanning and signal collection optics. However, high-contrast, nondistorted images also require the ability to adjust the laser power on various timescales, down to the microsecond level. This is a requirement for optimum performance in even simple, raster-scanned experiments. Laser power should be completely blocked as the microscope scan head returns the beam spot (flyback) for the next Y scan. It is even more important for applications scanning large z-stacks. As the system focuses deeper into the tissue, natural attenuation dictates the use of higher laser power to maintain constant image intensity. In increasingly sophisticated high-speed scanning protocols, power control can be used to maintain the optimum power level relative to the voxel dwell time.
High-speed and high-contrast power control over a wide wavelength range has proved challenging for both microscope manufacturers and a large proportion of users who build their own multiphoton microscopes — specifically, the problem of how to adjust the beam power in real time without unduly stretching (chirping) the pulse width or compromising the quality of the round, Gaussian beam required for high-resolution images.
The latest generation of ytterbium-based lasers now provides independent and fast power control of both the tunable beam and the fixed wavelength beam before they leave the laser head — in other words, with guaranteed pulse width and TEM00 beam quality (M2 < 1.1). At Coherent, acousto-optic modulators (AOMs) are used to provide total power control (TPC). The TPC feature can be externally controlled by a 0- to 10-V analog input, which is ideal for homebuilt microscopes, or via direct RF (radio frequency) input to the AOMs to minimize cost and complexity for multiphoton microscope manufacturers.
The laboratory of professor Bruno Weber at the University of Zurich develops in vivo imaging systems that study cell-to-cell communication in the cortex, and the interaction of neurons with the vascular system. This includes fast imaging of metabolic calcium transients. The lab recently acquired a TPC-equipped laser to further these studies. Figure 2 shows an early image from these studies.
Optogenetics
One of the most exciting new fields in life sciences research is optogenetics — a field that dovetails perfectly with the use of advanced microscopy to conduct functional studies in neuroscience. In optogenetics, light is used to trigger opsins (light-sensitive ion gates and pumps) expressed in cell membranes. In neuroscience experiments, this is used to stimulate, or sometimes silence, target cells in a population that is being studied. When combined with fluorescent imaging of functional indicators, such as calcium probes, this enables all-optical physiology experiments.
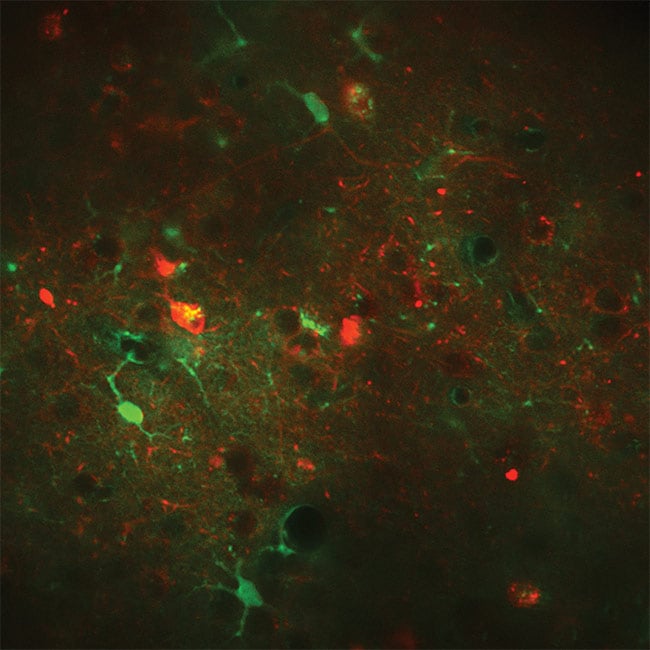
Figure 2. An example of fast frame rate calcium imaging. Overlay of neurons expressing RCaMP1.07 excited at 1100 nm (red) and astrocytes expressing GCaMP6s excited at 940 nm (green) in vivo in a mouse. Excitation source: Chameleon Discovery total power control (TPC). Courtesy of Weber Lab, University of Zurich.
In these microscopy studies, fluorescent imaging is used to map an area of the cortex and identify target neurons. Targeted neurons are then stimulated with light. The resulting activity in these neurons and the surrounding ones that are interconnected is mapped using fluorescence from calcium probes. If all of this imaging and stimulation is performed using multiphoton (typically two-photon) excitation, then the entire experiment can be carried out in a volume of live cortex with single-neuron resolution.
To minimize the crosstalk between stimulation and calcium interrogation, it is advantageous to use two well-separated wavelengths. Ytterbium-based ultrafast lasers offer the requisite wavelength options to perform multineuron studies based on established two-wavelength schemes using longer wavelength opsins such as C1V1, and a somewhat shorter wavelength calcium indicator such as Oregon Green. Equally well-suited alternative wavelength pairings are currently under development that are based on red-shifted calcium indicators of the GCaMP family.
Again, many researchers in this field are moving to larger populations of neurons, which require deeper and faster imaging. This creates a demand for higher power, but with a caveat. High pulse energy is needed to rapidly stimulate populations of tens or hundreds of neurons. However, the total laser energy loaded into the sample needs to be lower than the limits for nonlinear photo damage or excessive temperature elevation that would damage the cells. One solution is to use a ytterbium fiber amplifier with average power of tens of watts, but with exposure times of only a few milliseconds, and spread the energy over multiple neurons simultaneously with a spatial light modulator (SLM).
This need is met with a new generation of one-box ytterbium sources using a master oscillator/power amplifier architecture. This type of application is really in its infancy, however, with some researchers preferring repetition rates of 1 MHz, and others recommending repetition rates up to 10 MHz. Fortunately, ytterbium amplifier technology is extremely flexible and can cover this range and more. An example is the Monaco from Coherent, which delivers 40 W of output and with which the user can vary the pulse repetition rate from <1 to 50 MHz with no impact on beam properties (shape, size, and pointing).
Multiwavelength imaging
Fluorescence microscopy experiments that utilize continuous-wave (CW) lasers are becoming increasingly sophisticated and complex, with a common need to excite multiple probes in a single combined image. Many of these studies are performed with a confocal laser scanning microscope (CLSM) to provide 3D discrimination. Here, selectively exciting multiple probes means combining multiple lasers into the microscope.
And to support the latest probes, lasers at several new wavelengths are being developed. An interesting example is at 980 nm for microscopy relying on upconversion of nanoparticles (UCNP). This method successfully addresses the wavelength trade-off in deeper imaging with single photon microscopy. This overarching demand for an expanding range of wavelengths is met by using both laser diodes and optically pumped semiconductor lasers (OPSL). These complementary technologies are now offered in a common plug-and-play package such as the OBIS family of lasers from Coherent.
In terms of ease of use, a key challenge is to combine this multiwavelength functionality in a simple and robust manner that does not require significant laser expertise. Combining multiple lasers into a single mode fiber is one of most challenging optomechanical tasks in biophotonics. Typically, this has been done in a cumbersome serial manner using macroscopic, collimated beams, by first adding a second laser, then a third, and so on. This necessitates the cost and complexity of dichroic beamsplitters and other optics at each successive laser wavelength. Then the collinear beams have to be carefully coupled into the output delivery fiber, which usually requires many hours of work by a skilled optical technician using six-axis adjustable mounts. Moreover, the optical characteristics of the dichroic-coated optics are angle dependent and can result in significant losses. Additionally, the numerical aperture (NA) of conventional fiber varies with wavelength. The end result is a typical maximum optical throughput of only around 30 percent per laser.

Figure 3. Multiple lasers can be integrated in a single chassis, eliminating power supply and user interface redundancy. The fiber-coupled outputs can then be combined into one single-mode fiber using a plug-and-play beam-combining module such as the Coherent Galaxy. Courtesy of Coherent Inc.
In response to this limitation, engineers at Coherent have developed a completely different type of beam-combining module — the OBIS Galaxy. Based on simple refractive optics, this module accepts up to eight fiber inputs at wavelengths chosen by the customer — options include 405, 445, 458, 488, 514, 532, 552, 561, 588, 594, and 640 nm. Lasers are connected into Galaxy using standard (FC/UFC) inputs (Figure 3). The performance of this device is both stable and repeatable. Instead of hours of painstaking alignment, lasers can now be effortlessly replaced in minutes. The output of Galaxy is a standard FC/APC with extended life interface for superior reliability. Also available is a new FC/PC 8 output fiber connector compatible with spinning-
disk microscopy. Throughput is typically 75 percent for each laser.
For ultimate in convenience and ease of use, multiple fiber-coupled lasers can be mounted in a single housing. This offers all of the smart features from the laser in a convenient CDRH (Center for Devices and Radiological Health)-compliant interface, with convection cooling for five lasers — acting as a smart, integrated multiwavelength laser.
The synergism between applications and laser developments in optical microscopy is similar to many other examples across the life sciences. A new method is first demonstrated and proven, typically using a nonspecialized laser. The laser performance and its ease of use are then optimized to enable mainstream exploitation of the technique by the broadest possible user group.
Meet the authors
Darryl McCoy is director of product marketing at Coherent Inc., managing the ultrafast tunable and fiber laser products for nonlinear microscopy and instrumentation markets; email: [email protected].
Daniel Callen is a product manager at Coherent Inc. with over 25 years in the photonics industry and experience in manufacturing, engineering, and life science applications; email: [email protected].
Matthias Schulze is director of marketing OEM components and instrumentation at Coherent Inc. He received his Ph.D. in physics from the Technical University of Berlin; email: [email protected].
Marco Arrigoni is a director of strategic marketing at Coherent Inc. He covers the scientific research markets; email: [email protected].
Supporting Three-Photon Microscopy
A number of research groups want to extend the depth of multiphoton microscopy beyond the 1.5-mm-thick mouse cortex and into the underlying layers.
Several factors determine the maximum viewing depth with multiphoton excitation (MPE). In particular, scatter and absorption of the incident laser radiation must be carefully considered. As shown in the figure, the combination of these effects results in two windows in the near-IR where the penetration depth is maximized — at 1300 nm and in the 1700- to 1800-nm region.
These two regions are ideal for three-photon excitation of existing probes. But three-photon excitation efficiency depends on laser power ×3. This cutting-edge research is enabled by pumping a tunable parametric device, such as an optical parametric amplifier (OPA) or an optically pumped chirped pulse amplifier (OPCPA), with one of the new ytterbium fiber amplifiers.
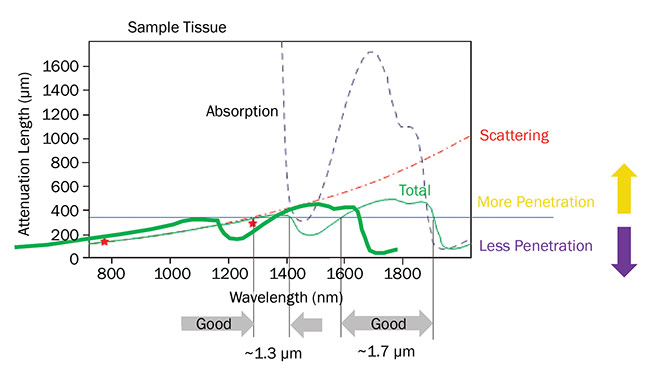
The penetration depth for ultrafast lasers in live brain tissue is limited by a combination of scattering and absorption. Two optimum penetration windows are centered at 1300 and
1700 nm. Based on a private communication with professor Chris Xu, Cornell University. Courtesy of Coherent Inc.