Recent advances in nanophotonics are improving live cell analysis and increasing the understanding of complex biological systems.
XIAOKANG LI, MARIA SOLER, FILIZ YESILKÖY, AND HATICE ALTUG, ÉCOLE POLYTECHNIQUE FÉDÉRALE DE LAUSANNE
In an era of fast-emerging personalized therapies, accuracy and efficacy rely mainly on precise cell analysis and engineering. With advanced gene sequencing technologies, researchers have revealed the complex heterogeneity in the genomics, proteomics, and epigenomics among cells that share the same phenotype. Decoding the behavior of individual cells is not only essential to fully understanding the underlying mechanisms, it also enables the screening and selection of potential candidates for innovative cell therapies.
Profiling the time course of a single cancer cell secretome, for example, provides insights into the disease progression. It also provides valuable information to analyze drug efficacy and identify potential cancer stem cells to reinforce the remedy. However, the precise investigation of individual cell behavior and functionality is still an arduous challenge in biomedical research, the pharmaceutical industry, and clinical laboratories1.
Live cell imaging techniques based on fluorescent labeling or in vitro immunoassays, such as ELISA (enzyme-linked immunosorbent assay), are the current tools for biomedical researchers to identify different cell types and functions. But these methods involve multiple molecular labeling processes that inevitably interfere with cell integrity and offer insufficient sensitivity and temporal resolution to attempt single-cell analysis in a real-time manner.
Illustration of a lymphoma cell as it adheres to nanohole arrays, which enables live cell analysis. Courtesy of the Laboratory of professor Hatice Altug/EPFL.
A recent study in the journal Small reports the development of a new nanophotonics technology for ultrasensitive and label-free monitoring of cell responses at the individual level2. The study’s researchers demonstrated the unique capabilities of the device for interrogating an isolated individual lymphoma cell and identifying its secretion activity in real time, all without disturbing the cell environment or introducing invasive agents. The work synergistically integrates a cutting-edge nanophotonic sensor based on plasmonics with an advanced microfluidics system to tackle the common problems and challenges faced by long-term optical monitoring of live cells.
Nanophotonics for biosensing
The last decade has seen a noticeable boost in the development of nanophotonic technologies for biosensing3. Myriad nanostructures and sophisticated assemblies have been fabricated to enhance the analytical sensitivity, reliability, and integration capabilities in miniaturized devices. Along with that, nanophotonic biosensors have become a benchmark for label-free and rapid biomolecular analysis with broad applicability as new diagnostic tools for rapid disease detection4–7, environmental monitoring, and food safety monitoring, among others8,9 (Figure 1). Surprisingly, this impressive technology has hardly been explored for live single-cell analysis, since monitoring complex biological processes in a label-free manner from an individual cell requires extreme sensitivity and superior system robustness to maintain cell viability and avoid interference that may lead to false positive or negative signals.
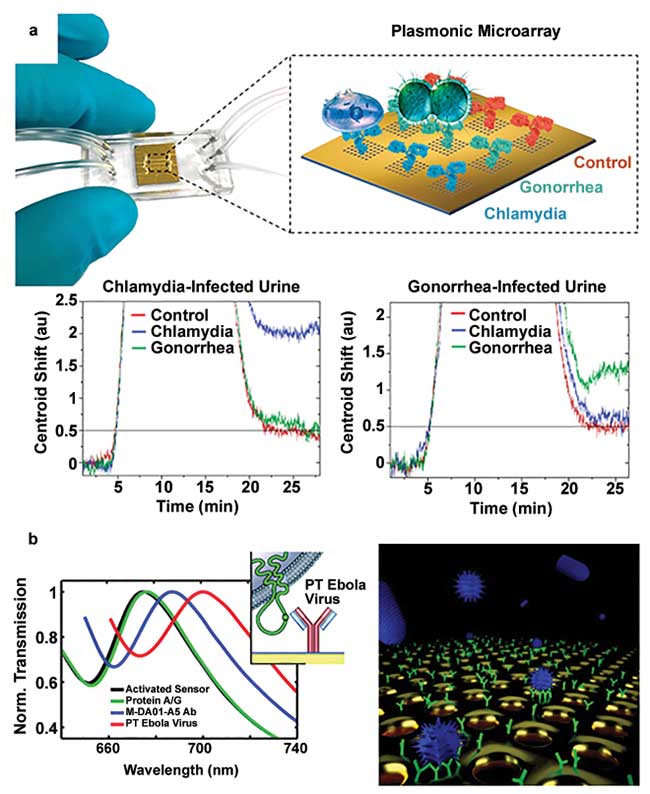
Figure 1. Examples of nanophotonic biosensors employed in disease diagnostics and environmental monitoring. A microfluidic-integrated nanoplasmonic biosensor was tested for simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine samples. The immobilization of specific antibodies on individual sensor arrays enables selective and multiplexed quantification of the bacteria in real time (a); adapted from reference 4. Demonstration of a nanoplasmonic biosensor for direct detection of vesicular stomatitis virus and pseudotyped Ebola virus. A dynamic range from 106 to 109 PFU/mL was shown corresponding to a clinical testing window relevant to drug screening (b); adapted from reference 6. PT: pseudotyped. Courtesy of École Polytechnique Fédérale de Lausanne.
A single cell can produce and secrete up to a few femtograms of cytokines (i.e., signaling proteins) after activation. These cytokines are relatively small biomolecules with molecular weight in the range of 15 to 30 kDa, which imposes a significant limitation for label-free detection based on refractive index changes. Quantifying minute amounts of specific small proteins from a complex sample (i.e., cell media) without amplification requires an exceptional sensitivity; it is only reachable by combining powerful nanotechnology with carefully designed surface chemistry procedures and effective microfluidic strategies. That aim has been accomplished in this work recently by introducing a solution involving cell deposition directly on the biosensor interface, a valve-gated microchamber for cell encapsulation, and a microfluidic system regulating temperature and humidity to ensure cell viability and stable device response over many hours.
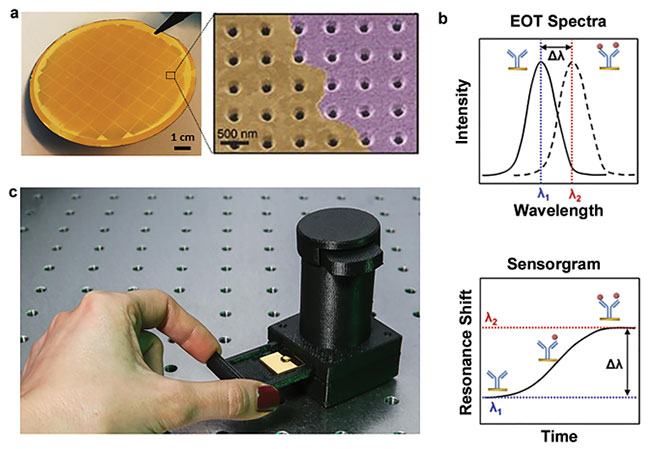
Figure 2. Detection principle of NHA-based nanoplasmonic biosensors. A fused silica wafer containing uniformly patterned plasmonic nanohole arrays with a hole diameter of 200 nm and an array period of 600 nm (a); adapted from reference 13. The EOT (extraordinary optical transmission) spectrum shifts upon accumulated mass on the nanohole array surface. By tracing the spectral shifts over time, a sensorgram is obtained showing the real-time biomolecular binding process that allows quantitative biodetection in a label-free manner (b); adapted from reference 2. A hand-held device measuring 7.5 cm high and weighing 60 g was developed to enable the nanoplasmonic biosensor for point-of-care diagnostics (c); adapted from reference 12. ROI: region of interest. Courtesy of École Polytechnique Fédérale de Lausanne.
The nanophotonic sensor consists of nanohole arrays (NHAs) fabricated on a thin gold film. These nanostructures can couple the normally incident light and generate surface plasmon polaritons (SPPs) that propagate across their surface and focus the electromagnetic field at the vicinity of the nanoholes (Figure 2a). This effect enables the so-called extraordinary optical transmission (EOT) phenomenon, which is characterized by the appearance of specific wavelength peaks on the transmitted light spectrum that are highly sensitive to mass changes on the nanoplasmonic surface10.
The versatility and robustness of nanophotonic sensors ... make them a general-purpose toolkit [for] biomedical and clinical sciences.
By interrogating the EOT peak displacements, one can monitor in real time biomolecular interactions occurring on the sensor substrate, similar to modern surface plasmon resonance (SPR) instruments (Figure 2b). The most interesting features of NHA sensors compared to other nanoplasmonic technologies are easy integration with almost all types of optical systems, from conventional microscopes to hand-held devices11,12 (Figure 2c), as well as a large field-of-view characterization that happens with the omission of the complex prism-based light coupling mechanism. The latter is especially relevant to facilitate the incorporation of sophisticated microfluidic designs for multiplexing purposes or, as in this case, for cell imaging and analysis. Additionally, they can be nanofabricated at extreme uniformity with wafer-scale and low-cost patterning techniques, such as deep ultraviolet (DUV) lithography13.
The NHA sensor has demonstrated an outstanding sensitivity, reaching limits of detection in the range of picograms per milliliter for small cytokines, including the vascular endothelial growth factor (VEGF)14 or interleukin 2 (IL-2)2. Nonetheless, monitoring the tiny amounts of cellular cytokine secretion remains a major challenge for label-free biosensors, and additional efforts are needed to address single-cell secretion analysis.
Biosensing strategies
A smart strategy is to enclose the active cells in miniature microfluidic chambers. By limiting the volume to a few nanoliters, the effective concentration of cytokines secreted by the single cells would increase over time, reaching the detection limits of the label-free biosensor for valid analysis. To realize this goal, the NHA sensor is assembled with a microfluidics device that incorporates a microsized channel with pneumatic valve gates (Figure 3a). The valves can be closed immediately after introducing the cells, which stops the flow and creates a microchamber where the cell attaches to the sensor surface and triggers cytokine secretion.
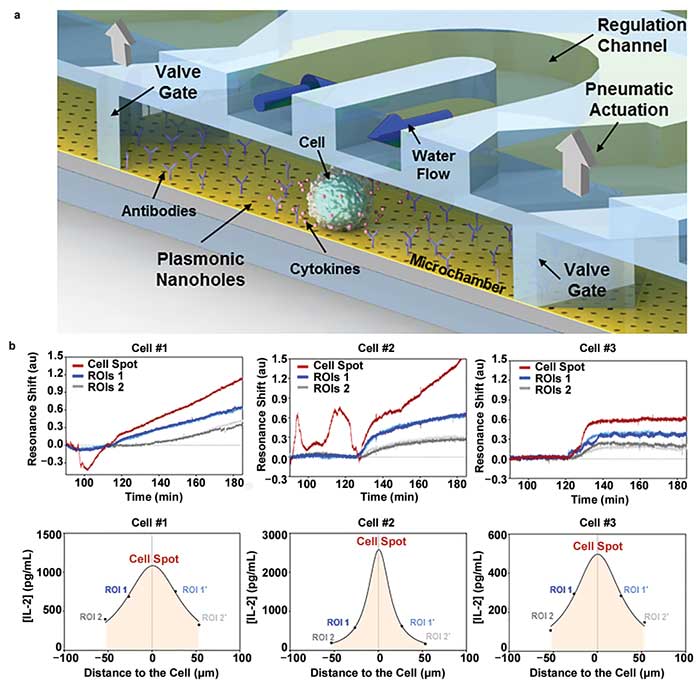
Figure 3. Optofluidic nanoplasmonic biosensor enables single-cell analysis. A schematic illustration of the microfluidic-integrated nanoplasmonic biosensor. The microfluidic system is composed of a primary channel where two pneumatic valve gates are included for enclosing a small-volume microchamber for single-cell analysis, and a regulation channel on top of the microchamber to provide environmental regulation via continuous water flow (a). A series of temporal/spatial label-free analyses of IL-2 secretion from three independent lymphoma cells, monitored simultaneously at multiple regions of interest (ROI), including at the cell spot and two nearby areas, 25 and 50 µm away from the cell spot (b). Adapted from reference 2. Courtesy of École Polytechnique Fédérale de Lausanne.
This biological process is not fast — it generally takes several hours for the cell to start releasing cytokines — and it requires appropriate cell culture conditions: 37 °C, 5 percent CO2, and high humidity. A customized incubator can be incorporated into the microscope to provide such environmental conditions. Furthermore, the microfluidic system can be entirely fabricated with polydimethylsiloxane (PDMS), a universal and affordable material that is biocompatible and permeable for gas exchange. However, when the flow stops at a temperature of 37 °C, a fatal problem arises: liquid evaporation.
A low volume of cell culture media under high temperature and strong light illumination will undoubtedly evaporate rapidly. This would not only kill the cells but also induce osmolality changes during evaporation, which directly affect the plasmonic sensor response, inducing a false signal. Perhaps the most straightforward solution is to immerse the biosensor in water tanks or place sacrificial water drops around it. But taking into account that the measurement is performed on a microscope with delicate optical and electronic components, this approach is far from optimal. Modern techniques, such as droplet microfluidics, deal with evaporation by immersing the isolated aqueous drops in mineral oil baths. Although this strategy is effective, it can be complicated and difficult to integrate in lab-on-a-chip devices because of contamination problems with the assay.
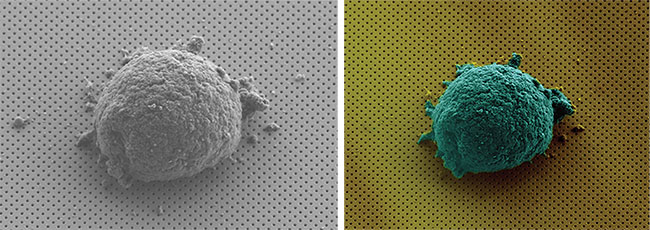
Different views of a single lymphoma cell adhering to nanohole arrays. This enables the label-free, single-cell secretion analysis of live cells. Courtesy of the Laboratory of professor Hatice Altug/EPFL.
The approach introduced in this work is inspired by traditional laboratory tools and household appliances — the incorporation of a water flow regulation channel in close contact with the microchamber to provide high humidity, minimize the relative vapor gradient, and significantly attenuate evaporation. This is similar to the flux condensers found in distillers, refrigerators, and cars, but miniaturized and integrated into a microfluidic system.
With such an optofluidic nanophotonic biosensor, an isolated immune cell can be monitored over several hours for real-time detection of the activation and protein secretion processes without any labels. The unique design of this nanoplasmonic chip with nanohole arrays extending across the entire sensor surface provides complete flexibility in defining multiple regions of interest around the sedimenting single cell to spatially and temporally map the spectral fingerprint of the cytokine secretion (Figure 3b). This subcellular resolution and label-free assay format could advance the capabilities of nanoplasmonic cell analysis beyond the current state of the art.
The versatility and robustness of nanophotonic sensors, along with their ability to detect a large variety of biomolecules, make them a general-purpose toolkit with various applications in biomedical and clinical sciences. The fields of immunology and oncology can readily benefit from such a system to carry out accurate analysis and evaluation of cell functionalities or drug efficiency. In the meantime, fundamental biology researchers can exploit this lab-on-a-chip device to get significant insights and comprehensive information about extracellular signaling and relevant cell biology processes.
Meet the authors
Hatice Altug, Ph.D., is a professor at the École Polytechnique Fédérale de Lausanne Institute of Bioengineering, leads the Bionanophotonic Systems Laboratory, and is director of the EPFL Photonics Doctoral School. She has a Ph.D. in applied physics from Stanford University; email: [email protected].
Xiaokang Li is a Ph.D. student in the Bionanophotonic Systems Laboratory at École Polytechnique Fédérale de Lausanne. He has bachelor’s and master’s degrees in biomedical engineering from Tsinghua University in China; email: [email protected].
Maria Soler, Ph.D., is a senior researcher at the Catalan Institute of Nanoscience and Nanotechnology (ICN2) in Spain, where she received her Ph.D. in biochemistry and biomedicine. She is a former postdoctoral scientist in the Bionanophotonic Systems Laboratory at École Polytechnique Fédérale de Lausanne; email: [email protected].
Filiz Yesilköy, Ph.D., is a postdoctoral researcher in professor Altug’s laboratory at École Polytechnique Fédérale de Lausanne, where she held a similar post in professor Brugger’s laboratory. She is also a former guest researcher in professor Kim’s group at the University of Tokyo. She has a Ph.D. in electrical engineering from the University of Maryland, College Park; email: [email protected].
References
1. G-C. Yuan et al. (2017). Challenges and emerging directions in single-cell analysis. Genome Biol, Vol. 18, article 84.
2. X. Li et al. (2018). Label-free optofluidic nanobiosensor enables real-time analysis of single-cell cytokine secretion. Small, Vol. 14, Issue 26.
3. G. Lopez et al. (2017). Recent advances in nanoplasmonic biosensors: applications and lab-on-a-chip integration. Nanophotonics, Vol. 6, Issue 1.
4. M. Soler et al. (2017). Multiplexed nanoplasmonic biosensor for one-step simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine. Biosens Bioelectron, Vol. 94, pp. 560-567.
5. H. Im et al. (2014). Label-free detection and molecular profiling of exosomes with a nanoplasmonic sensor. Nature Biotechnol, Vol. 32, pp. 490-495.
6. A. Yanik et al. (2010). An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett, Vol. 10, pp. 4962-4969.
7. J. Jackman et al. (2016). Plasmonic nanohole sensor for capturing single virus-like particles toward virucidal drug evaluation. Small, Vol. 12, Issue 9, pp. 1159-1166.
8. K-L. Lee et al. (2016). Nanoplasmonic biochips for rapid label-free detection of imidacloprid pesticides with a smartphone. Biosens Bioelectron, Vol. 75, pp. 88-95.
9. J-H. Park et al. (2014). A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A. Biosens Bioelectron, Vol. 59, pp. 321-327.
10. C. Escobedo. (2013). On-chip nanohole array based sensing: a review. Lab on a Chip, Vol. 13, Issue 13, pp. 2445-2463.
11. A. Coskun et al. (2014). Lensfree optofluidic plasmonic sensor for real-time and label-free monitoring of molecular binding events over a wide field-of-view. Sci Rep, Vol. 4, pp. 6789.
12. EPFL News. (2014). A Complete Medical Check-up On a Chip. https://actu.epfl.ch/news/a-complete-medical-check-up-on-a-chip/.
13. F. Yesilkoy et al. (2018). Phase-sensitive plasmonic biosensor using a portable and large field-of-view interferometric microarray imager. Light Sci Appl, Vol. 7.
14. X. Li et al. (2017). Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip, Vol. 17, Issue 13, pp. 2208-2217.