Powered by laser technology, the technique has been used to rapidly identify cancer and fibrosis in tissues and could aid in guiding therapeutics.
TAYLOR STATHOPOULOS AND JEREMY ROWLETTE, DRS DAYLIGHT SOLUTIONS
As microspectroscopic imaging applications have expanded, analytical scientists have demanded advancements from existing imaging technology. Such advancements are particularly critical for biomedical applications, such as tissue analysis in cancer research, where accuracy and speed are crucial for the adoption of new imaging systems. Clinical demands have pushed researchers and industry to develop new, powerful instrumentation, such as quantum cascade laser (QCL)-IR microscopes, that can analyze large volumes of tissue samples rapidly.
Courtesy of iStock.com/sudok1.
To address the growing challenge of rising cancer rates, for instance, researchers continue to seek out more sophisticated techniques and technology for identification and treatment. The International Agency for Research on Cancer reported that in 2018 there were 9.5 million cancers deaths and 17 million new cancer cases worldwide, encompassing all types of cancer. The agency predicts that by 2040, new cancer cases will grow to 27.5 million and cancer deaths will climb to 16.3 million per year, due to the growth and aging of the population.
Aiming for faster results, cancer researchers have trended toward novel techniques and technologies that add high throughput without sacrificing data quality or patient care. A variety of techniques involving both spectroscopy and microscopy, such as QCL-IR microscopy, are finding increasing use by researchers and are helping to improve patient outcomes.
Chemical mapping
In general, molecules strongly absorb IR light. Each individual molecule exhibits a unique spectral fingerprint that allows it to be identified, imaged, and quantified using IR spectroscopic techniques. IR spectrometry was conceived at the turn of the 20th century and has since been continually enhanced. The Fourier transform IR (FTIR) spectrometer was developed in the 1960s. FTIR has been widely adopted because of its comparatively high signal-to-noise ratio (SNR) and broad IR wavelength coverage. But while FTIR provides IR spectra, it does not provide information about the spatial distribution of chemicals (molecules) within a sample. The demand for spectral and spatial information led to the development of the FTIR microscope.
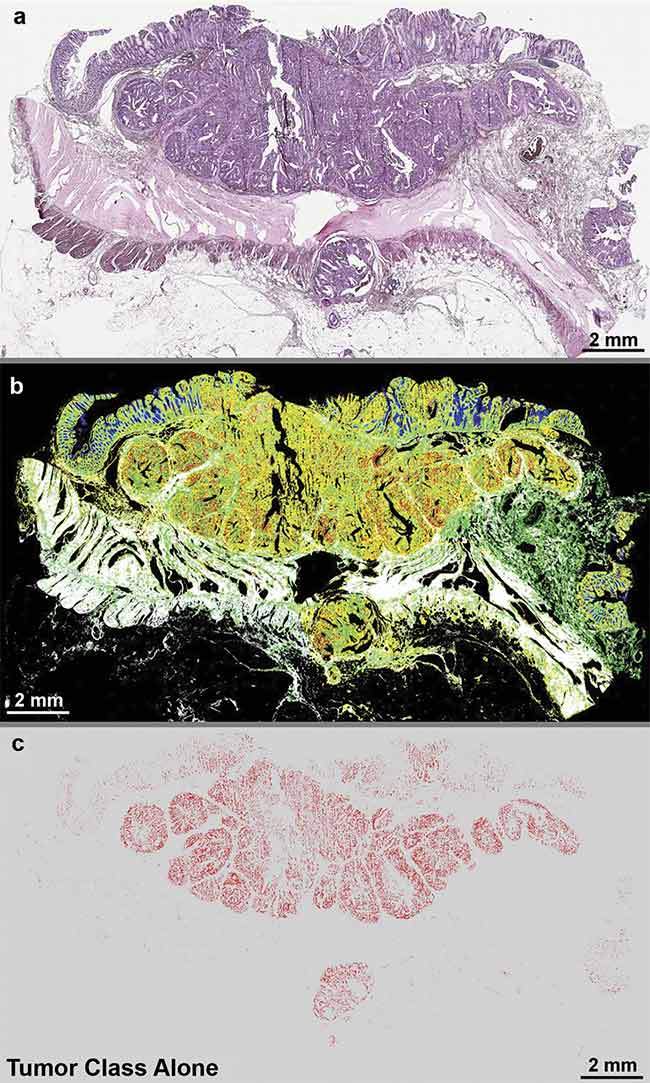
Quantum cascade laser (QCL)-based IR imaging of whole slice colorectal cancer tissue thin sections. The tissue was stained with hematoxylin and eosin (H&E) (a), and label-free images were taken using a QCL-IR microscope (b, c). The H&E image shows the structure of the diseased colon wall (a), while (b) highlights the classes of tissue and (c) highlights the tumor alone. Adapted with permission from Reference 1/CC BY 4.0.
IR microscopy provides researchers with a chemical map of a sample, including aspects that are not visible under a white-light microscope. Crucially, IR imaging does not require that the sample be stained or dyed (labeled) to reveal the presence of molecules of interest. This allows samples to be preserved and to undergo other types of analysis, if needed.
In the case of cancer research, these chemical maps can — with the help of advanced software algorithms — be used to detect the presence of cancerous tissue and identify the cancer types and subtypes. Similarly, they can be used to identify and quantify microplastics in water samples — another important application area. For these reasons, FTIR microspectroscopy became the technique of choice for the chemical imaging of tissue (histology) and histopathology applications.
FTIR microscopes, however, have limited data acquisition speeds due to their use of relatively low-brightness Globar incoherent light sources. A Globar is a thermal source that emits photons over a broad spectral range and a wide angle, somewhat like the IR equivalent of an incandescent light bulb. The use of Globar light sources requires the operation of a sensitive, liquid-nitrogen-cooled detector and a scanning interferometer (moving mirror) to detect the IR signal and build up an image of the sample, point by point, a process that is relatively slow.
So, while FTIR technology provides a novel approach with which to characterize tissue, it lacks the efficiency needed for it to be adopted in pathology labs, where tens to hundreds of samples may be imaged daily. This drove the development of a faster infrared microscope platform — the QCL-IR microscope.
QCL-IR microscopy addresses the demand for high-throughput, high-
sensitivity microspectroscopy. The unique advantage of this approach is attributable to the specific characteristics of the quantum cascade laser source inside. QCLs emit high-spectral and high-spatial-brightness mid-infrared light that is orders of magnitude brighter than the Globar used in FTIR microscopes. This allows images to be captured more rapidly and with high SNR.
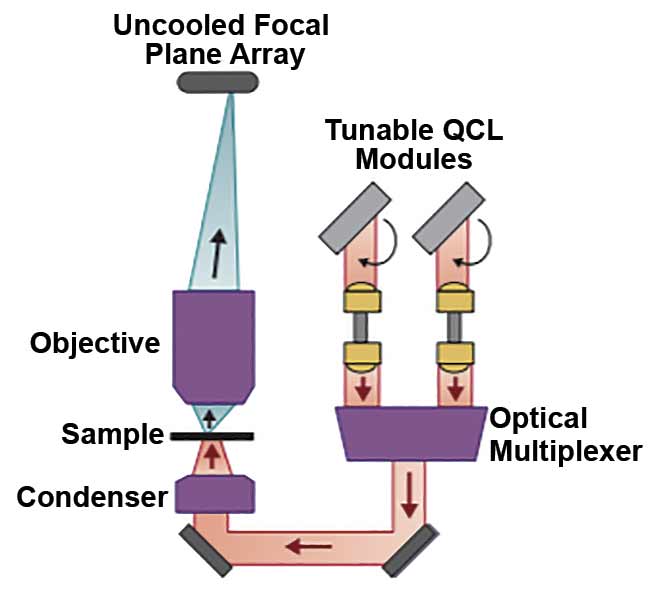
The subsystems in a QCL-IR microscope. At any given instant, only a narrow wavelength (wave number) band emits from the QCL source. The exact wavelength of this laser is precisely controlled by the actuation of an external cavity frequency-selective element (a diffraction grating) at a rapid tuning speed (microseconds). The laser light is then transmitted (in the case of transmission imaging) through the sample, and then it passes through a wide-field infrared objective before
it is collected by the focal plane array imager.
Courtesy of DRS Daylight Solutions.
The higher brightness of QCLs enables the use of an uncooled IR focal plane array to capture the entire field of view. This enables wide-field IR imaging without the need for point-by-point scanning. Commercial embodiments of the QCL-IR technique, such as the Spero chemical imaging microscope, couple high QCL brightness with diffraction-limited compound refractive objective lenses to illuminate hundreds of thousands of pixels simultaneously (in parallel). This proprietary approach enables extremely high-throughput chemical imaging without sacrificing sensitivity or image quality. This allows users to collect a chemical image up to 150× faster than a conventional FTIR microscope at an equal SNR. While FTIR offers broader spectral coverage than QCL-IR, the success of QCL-IR microscopy in a variety of applications has shown that this wider coverage is not essential for many applications, including histopathology.
Benefits of QCL-IR
Researchers are already imagining a future in health care aided by QCL-IR microscopy, and some have already published and presented research expounding on the potential clinical application of this technology. While QCL-IR microscopy is attracting considerable interest for transforming tissue analysis, it is also being explored as a novel way to examine metabolic pathways, examine the effects of therapeutics on tissue, and determine disease progression.
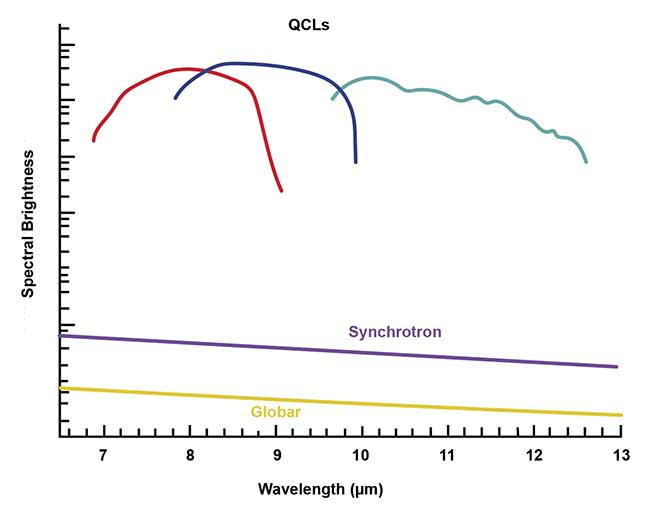
When used in Fourier transform-IR microscopes, QCLs produce significantly more brightness than Globar or synchrotron light sources. Courtesy of DRS Daylight Solutions.
For both patients and doctors, the most critical parts of a cancer protocol involve developing a treatment plan and determining disease progression. For a treatment plan to be successful, the doctor must consider the characteristics of the diseased tissue, including mutations. Lung cancer, for example, can manifest as many different subtypes, each requiring very different treatments.
The gold standard of cancer analysis requires histological staining of tissue, typically using hematoxylin and eosin (H&E), to allow differentiation of diseased tissue. Highly skilled pathologists analyze the stained tissue under a white-light microscope to observe morphological changes and decode the results of the staining. This method is broadly used for routine cases, but it lacks the robust, quantitative information needed for making decisions in challenging cases. Without this qualitative information, increased stress is placed on the already overburdened pathologists to make personal judgments about cases and spend several hours running follow-up tissue studies.
A German research team led by professor Klaus Gerwert at Ruhr University Bochum, with its development of a simplified and automated approach using QCL-IR microscopy, is looking to advance current cancer protocols. The team has collaborated with pathologists and oncologists to collect quantitative chemical imaging information on cancerous tissue much more rapidly. QCL-IR microscopy is ideally suited for this kind of analysis because it can provide rich hyperspectral data in under 1 min for a 2- × 2-mm microscope field of view.
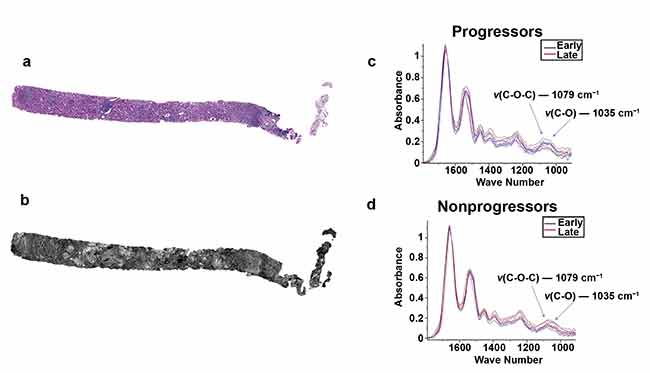
Two serial sections of tissue were placed on a glass slide and an IR-compatible substrate. One section was stained with periodic acid-Schiff (a) while the other unstained section was imaged using QCL-based IR imaging (b). A region of interest was located and biochemical data was collected for progressor (c) and nonprogressor (d) cohorts for both early- and late-time-point biopsies. Adapted with permission from Reference 2/CC BY 4.0.
In a recent publication, these researchers coupled QCL-IR microspectroscopic data with advanced machine learning and AI image analysis to demonstrate automated IR cancer tissue classification. In a study of 110 tissue samples from stage 2 colorectal cancer patients, results indicated 100% selectivity and 96% sensitivity compared to classical histopathology1.
Therapeutics
The study of metabolic pathways — the series of chemical activities that keep cells functioning — is the future of determining pathologies, monitoring disease progression, and analyzing the effects of therapeutics. For such studies to be effective, there is a need for quantitative, rapid analysis of metabolic image data.
A novel approach to studying metabolic pathways using infrared imaging takes advantage of the “naked band” of the mid-infrared. At around 1800 to 2700 cm−1, there is a spectral region where biological samples exhibit no infrared absorption. Researchers from Columbia University developed a specialized small-molecule IR probe that exhibits absorption in this band. By tagging samples with the probe and imaging the parts of the samples that exhibit its IR signature, the researchers were able to study metabolic pathways in situ.
In addition to helping develop earlier-stage and more accurate disease information, QCL-IR imaging research is helping to elucidate the effects of therapeutics in treating disease. Researchers from the University of Illinois, led by professor Michael J. Walsh, have leveraged QCL-IR microscopy to discover biomarkers for fibrosis progression in renal transplant patients. According to the Centers for Disease Control and Prevention, kidneys are one of the most transplanted organs in the U.S. Renal transplant surgery is a life-saving procedure, but it carries risks from several potential complications, including the development of blood clotting and fibrosis.
Fibrosis is a type of scar tissue that causes structural damage to connective tissue in organs such as the kidneys and can potentially cause renal failure due to an inability to heal. A biochemical marker for fibrosis could identify patients who are at risk for fibrosis — even when the post-operative biopsies of such patients appear histologically normal. Identifying at-risk patients would allow for early intervention if fibrosis does develop, which could help to slow disease progression. The University of Illinois study demonstrated that QCL-IR microscopy was able to find biochemical differentiation between patients with rapid progressive fibrosis and those without.
Digital pathology
In pathology, there is demand for digitization of analytical processes, or digital pathology. This demand is being driven by pathologists to increase process efficiency and data quality. The current pathology protocol often requires that glass slides be sent to labs or clinics, risking the chance of broken slides and damaged samples, and it incurs waiting times based on varying shipping speeds.
Many believe the future of pathology will involve adopting more advanced automation and artificial intelligence into the analytical process. Rapid-scanning glass scanners, for example, can upload high-resolution data into cloud-based servers. This allows data to be shared rapidly, ensuring fast and secure delivery of tissue sample data. In 2017, the FDA approved the first whole-slide scanner imaging system, developed by Philips. Although these scanners continue to use traditional stains, their introduction emphatically signals the criticality of automation and AI to the future of pathology. While these whole-slide imaging systems are being adopted in clinics for the traditional analysis of H&E-stained tissue, the research community continues to question the relevance of chemical stains and labels.
QCL-IR microscopy offers great potential, especially in a clinical setting, to eliminate the need for sample staining and enable the completion of multiple tests in one step. Eliminating this need would open the door for QCL-IR technology to be used in a wide range of environments, benefiting medicine and research.
Meet the authors
Taylor Stathopoulos is sales and marketing manager at DRS Daylight Solutions, where she is responsible for managing and executing integrated marketing communications strategies. She holds a Bachelor of Science degree in marketing from San Diego State University and is currently pursuing a Master of Science degree in marketing at Johns Hopkins University; email: [email protected].
Jeremy Rowlette, Ph.D., is senior director of the scientific and life sciences product lines at DRS Daylight Solutions. Prior to working at DRS Daylight, he was a senior development engineer at Intel Corp. He holds a doctorate in electrical engineering from Stanford University; email: [email protected].
References
1. C. Kuepper et al. (2018). Quantum cascade laser-based infrared microscopy for label-free and automated cancer classification in tissue sections. Sci Rep, Vol. 8, No. 7717, www.doi.org/10.1038/s41598-018-26098-w.
2. V. Varma et al. (2018). Predicting fibrosis progression in renal transplant recipients using laser-based i.nfrared spectroscopic imaging. Sci Rep, Vol. 8, No. 686, www.doi.org/10.1038/s41598-017-19006-1