
Ultrafast Lasers Excite Advancements in Multiphoton Imaging
The next generation of ultrafast lasers is enhancing multiphoton microscopy by penetrating deeper into biological tissue and generating higher-resolution images.
CHRISTOPHER LEBURN, CHROMACITY LTD., AND CHRISTIAN WILMS, SCIENTIFICA LTD.
Multiphoton microscopy can image deeper into tissue than traditional confocal microscopy, making it ideal for a range of life science studies. The technique is crucial for brain-related research because it allows imaging of large numbers of neurons or individual synapses and can also be performed in live animals. Today, most multiphoton imaging research employs two-photon techniques in which fluorescent dyes that emit light upon excitation are used.
Commonly, a fluorescence molecule is promoted to an excited state by absorbing a single excitation photon. But two photons of half the energy required for excitation can also excite the fluorescence molecule — if they interact with that molecule on an attosecond timescale. Achieving the necessary density of excitation photons requires the use of ultrashort-pulse lasers. Once a molecule is in an excited state, it relaxes back to the ground state, emitting a fluorescence photon.
The advantage of this technique over single-photon imaging is that it allows the generation of fluorescence signals in very small volumes, which provides images with higher resolution and contrast and little to no out-of-focus light as the laser beam is scanned over a sample. The long wavelengths used to generate two-photon signals also permit noninvasive cellular imaging deeper into tissue, to depths between 500 and 700 µm. The two-photon imaging market is currently served by both fixed-wavelength and tunable light sources. Both approaches have their place in the world of multiphoton imaging.
Tunable Ti:sapphire laser technology can span the excitation bandwidths across multiple fluorescent markers, but developments in fixed-wavelength ultrafast lasers enable provision of higher powers across several wavelengths and at lower cost.
Tunable sources
Multiphoton imaging has relied upon tunable Ti:sapphire lasers since the early 1990s. These diode-pumped solid-state systems can generate ultrashort sub-100-fs pulses and correspondingly broad bandwidths, providing the ability to excite a wide range of fluorescent markers via the two-photon process.
While they do not offer particularly high pulse energies or easily adjustable repetition rates, Ti:sapphire laser sources remain a popular choice for two-photon microscopy due to their low noise and the inherent flexibility that tunability offers. Ranging from common UV-excited fluorophores through to the orange and red dyes that fluoresce at around 1 µm, Ti:sapphire lasers work well across a wide range of application fields.
There are, however, some important limitations when it comes to using tunable sources in microscopy.
As a diode-pumped solid-state laser technology, Ti:sapphire sources provide acceptable power and beam quality, but they are inherently complex and require an expert to install them — a process that typically can take two or three days.
In addition to being very expensive, Ti:sapphire lasers are also bulky and costly to run, due to the high energy required to excite the laser gain crystals. Historically, these systems have had a poor mean time to failure. Having a free-space cavity requires critical submicron alignment, and, therefore, a lot of time, effort, and cost goes into ensuring that these optical cavities remain aligned.
Over the years, design improvements have enabled electronic control of the cavity to make Ti:sapphire systems significantly more user-friendly. However, these systems continue to occupy a large space on the optical bench due to their need for ancillary equipment, such as pulse precompression and power control. They also need a water-cooling system, which further contributes to higher operational running costs and workspace constraints, and also has a negative impact on the environment. Ti:sapphire lasers can produce heat loads up to 2.5 kW, which then need to be factored into the air-conditioning requirements of the research lab.
A standard Ti:sapphire laser may cost around $160,000. Adding all the ancillary components and consumables needed to perform life science imaging experiments can push the cost beyond $250,000.
Factor into this the cost of service contracts that, while necessary, can easily push the overall cost of ownership to more than twice the purchase price of the laser over 10 years, which often puts these systems well beyond the reach of many life scientists.
Life science imaging experiments increasingly require additional functionalities, such as dispersion management or the ability to switch a laser on and off very quickly. Applying these features to a fully tunable laser system is challenging.
The simultaneous excitation of multiple markers is another important functionality in current brain research, including
study of the activity of individual neurons. While Ti:sapphire lasers are able to emit a broad range of wavelengths, they can only produce one laser line at a time. So multiple markers cannot be observed simultaneously.
This has prompted increasing interest in alternative laser technologies that may not offer the same range of wavelength coverage but still provide high-quality imaging capabilities at key wavelengths.
Fixed-wavelength sources
For applications that do not require broad bandwidth tunability, recent developments in fixed-wavelength, fiber-based architectures have prompted the emergence of laser systems that are more efficient, stable, and robust than Ti:sapphire-based technologies. Typical wavelength regions covered by such systems center around 1040 and 920 nm — both of which can excite many of the most commonly used fluorescent markers, such as eGFP (enhanced green fluorescent protein), RFPs (red fluorescent proteins), and all commonly used genetically encoded calcium indicators, including GCaMP, R-GECO, and RCaMP.
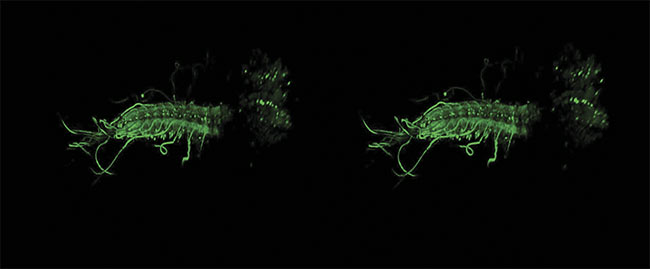
A stereo image of an intact ex vivo central nervous system of a Drosophila melanogaster larva, with GCaMP6m expressed in all motor neurons. Image acquired by James Macleod, with support from professor Stefan Pulver and Cold Spring Harbor Laboratory, using a Scientifica HyperScope. Courtesy of James Macleod.
Solid-state laser systems typically have to be overengineered to make them stable enough for today’s imaging requirements.
Fixed-wavelength fiber lasers are inherently more reliable and compact compared to Ti:sapphire technology. They are optically pumped by standard telecom-rated laser diodes that are more robust, efficient, and cost-effective, as well as a fraction of the size of the solid-state diodes required to pump Ti:sapphire lasers. Furthermore, these diodes pump a gain material contained within an optical fiber, which negates the complex optical alignment issues associated with Ti:sapphire systems. These features allow fiber-based fixed-wavelength sources to have shoebox-size footprints.
Life science researchers are able to set up fiber-based lasers themselves in under an hour, often with online remote support. The simplicity of these systems, coupled with their stability, means that once fixed-wavelength fiber lasers are up and running, they require very little additional attention from users. This obviates the need for the expensive service packages that solid-state systems require and it also lowers the total cost of ownership even further.
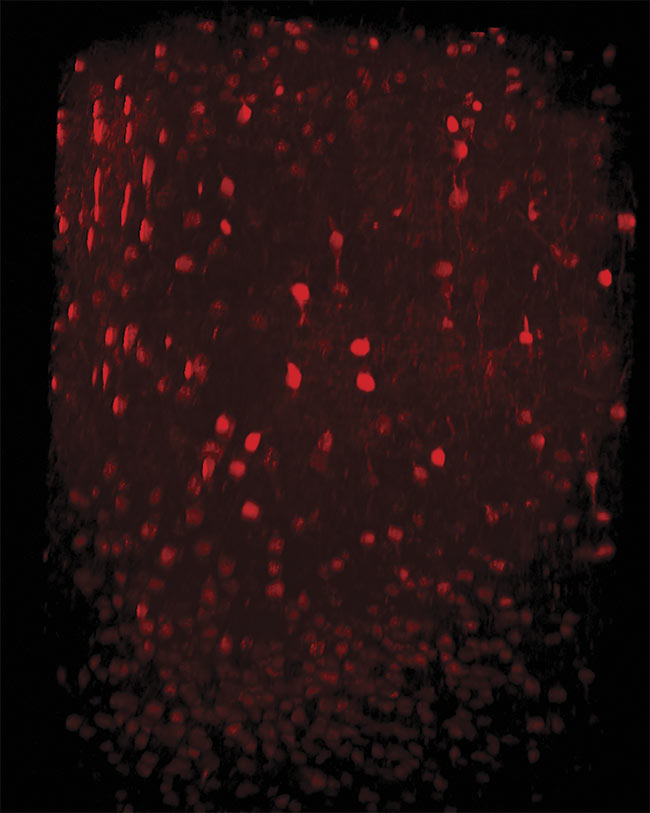
Three-photon imaging of interneurons in the mouse neocortex. Neurons are labeled with tdTomato expressed under the vesicular GABA transporter (VGAT) promoter. An imaging depth of 1000 µm was achieved with the dura left intact. Image generated using the HyperScope with an Avus and Satsuma hp2. Courtesy of Dr. Pedro Garcia da Silva.
The improved efficiency of fiber laser architecture also reduces thermal demand. Many of today’s fiber-based systems do not require water cooling. This can be an important factor in small lab spaces, where the noise produced by water-cooled systems can make a working environment uncomfortable. In addition, the heat loads produced by fiber laser systems are only around 200 W versus
the 2.5-kW heat loads of Ti:sapphires. This often means that the main heat source in the lab is actually the computer workstation.
Even before water cooling is factored out, the overall footprint of fixed-wavelength fiber lasers is much smaller than that of Ti:sapphires. Elements such as power control and pulse precompression are built into the fixed-wavelength fiber lasers’ streamlined, compact housing.
Crucially, fixed-wavelength laser sources also have higher average powers
than entry-level tunable Ti:sapphire
systems — on a scale of 4 W versus 1.5 W
or less, respectively. This leads to interesting application benefits. High-power tunable options are available at certain wavelengths, but at a price point that is typically out of reach for the majority of researchers.
Applications and modalities
To study biological samples, researchers make use of fluorophores — molecules that absorb light of a specific wavelength and emit it at a longer wave-
length. In the two-photon excitation regime, fluorophores typically have large excitation bandwidths, which often
allows fixed-wavelength sources to excite a range of labels.
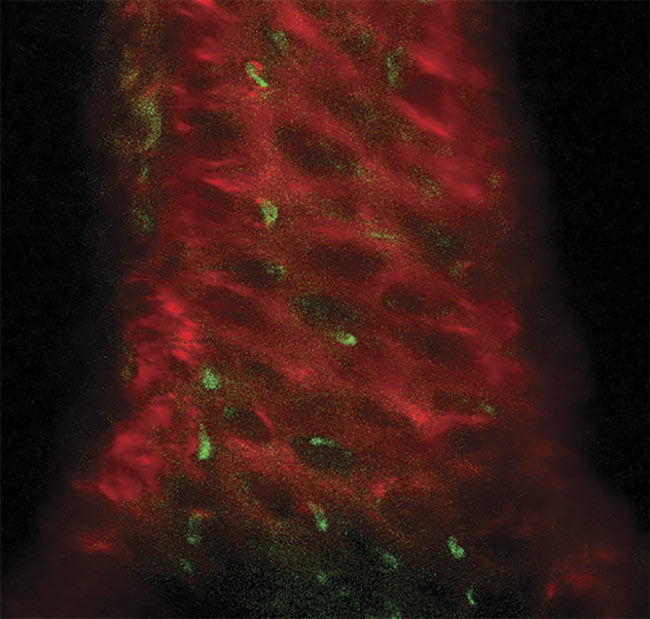
A three-photon image of an ex vivo mouse sternum, taken 200 µm deep using the HyperScope imaging system. Second-harmonic generation (SHG) from the bone structure (red) and three-photon excited eGFP (enhanced green fluorescent protein)-labeled nestin-positive cells (green). Image generated using the HyperScope with an Avus and Satsuma hp2. Courtesy of Dr. Tobias Ackels/Francis Crick Institute.
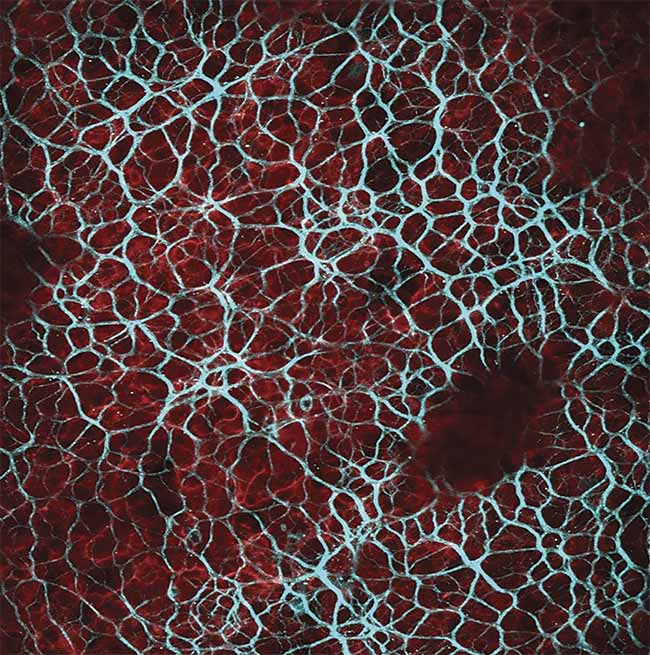
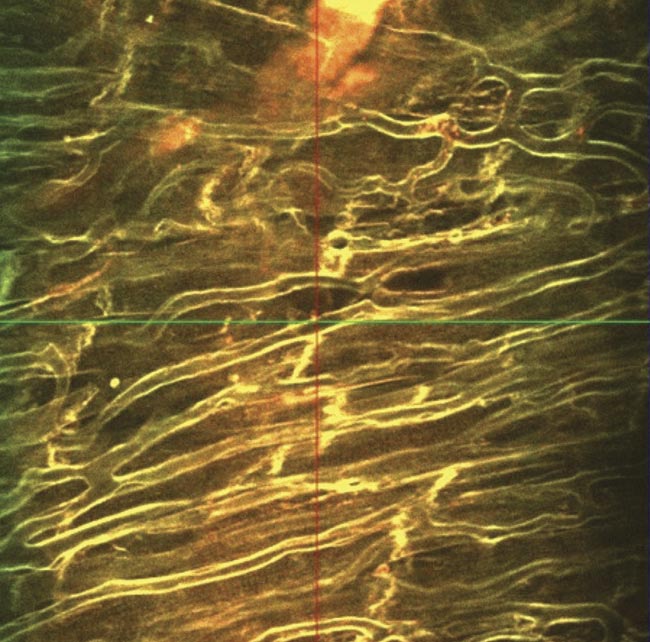
Results acquired when imaging with a fixed-wavelength laser source. Multimodal imaging in which liver cells overlaid by collagen tissue can be differentiated using mT:mG as a marker within the liver cells (red) and an SHG signal from collagen fibers (blue) (top). Ex vivo z-stack imaging at the epicardial surface of a mouse’s left ventricle, in which the tissue is stained with di-4 ANEPPS (bottom). Cardiac tissue could be imaged at depths of 250 µm. Image acquired using the Chromacity 1040. Courtesy of Institute of Genetics and Molecular Medicine/University of Glasgow.
Because of this, much of today’s research does not necessarily require
multiple excitation laser lines. Instead, two main wavelength regions are typically targeted between 1000 and 1100 nm
or between 890 and 960 nm. The first
fiber-based lasers started out with emission wavelengths between 1040 and
1060 nm. A new generation of sources, however, is becoming available that
covers the 920- to 950-nm region.
Neuroscience is a good example of a field in which fixed-wavelength lasers can play a key role. The majority of multiphoton microscopy users in this field are interested in studying the activity of neurons, which they do by observing a fluorescent protein-based indicator that lights up when neurons are active. These types of experiments require a system that can be set up quickly, and run reliably during multiple shifts, which often equates to 20 hours a day. By using two fixed-wavelength sources, or one in combination with a Ti:sapphire laser, researchers can also achieve the ability to simultaneously excite fluorophores at different wavelengths.
While solid-state lasers were once thought of as superior with regard to optical noise, recent advancements in fiber-based systems have enabled comparable performance — certainly from a life science imaging perspective.
Fiber laser technology has demonstrated its capabilities in two-photon fluorescence microscopy across a range
of samples, using an ultrafast, fixed-wavelength, 1040-nm ytterbium laser source. Its ultrashort-pulse duration is ideal for generating a label-free second-harmonic response from noncentrosymmetric molecular structures, such as collagen and starch.
In experiments involving the imaging of cardiac tissue, the power levels from fixed-wavelength sources were proven to be more than adequate for generating
fluorescence images of comparable quality to those generated by Ti:sapphire sources. In addition, the experiments showed that the higher power of the fiber laser, combined with its ability to adjust dispersion to maximize fluorescence, permitted greater depth penetration by 50 to 100 µm when compared to the tunable system. Along with the advent of new laser lines, such as 920 nm, to complement those of 1040-nm sources, these fiber-based systems are improving the ability to achieve exciting discoveries in biomedical imaging.
While two-photon microscopy is set to remain a key tool in life sciences research for many years to come, there is increasing interest in three-photon techniques, in which three lower-energy photons must be simultaneously absorbed to achieve the excitation of a fluorescent molecule.
Three-photon microscopy
The step from two- to three-photon excitation brings two advantages that allow deeper imaging with better contrast.
First, tissue scatters the longer-wavelength light less, which enhances the focal volume and allows for more efficient excitation in deep tissue. Second, the requirement for three photons to interact with the fluorescent molecule at the same time drastically reduces the risk of out-of-focus fluorescence.
While two-photon microscopy does not suffer from out-of-focus fluorescence background in superficial tissue, using it to image deep tissue can lead to a reduction in image contrast. In mouse brain tissue from a depth of ~500 µm onward, three-photon imaging has proved to yield superior contrast as a result of its better focal confinement.
As a result, three-photon microscopy enables noninvasive high-contrast imaging of cells at depths >1 mm. This is of substantial interest to research in regenerative medicine, cancer, plant sciences, and in the study of Alzheimer’s disease.
Creating three-photon excitation re-uires high photon densities and, thus, high pulse energies and peak powers. Therefore, the technique requires femtosecond sources that typically operate with pulse repetition frequencies below 4 MHz
across the near-infrared spectrum. Average power levels must be kept to a minimum to avoid damaging tissue with heat.
While three-photon excitation systems based on optical parametric amplifiers (OPAs) are available, they are prohibitively expensive and highly inefficient — providing less than 10% total conversion efficiency.
Work is underway to develop commercially viable alternatives that are compatible with existing microscopy platforms. The goal is to develop cost-efficient lasers for three-photon microscopy using low-megahertz optical parametric oscillators (OPOs) that are pumped by ultrafast fiber lasers. In contrast to OPAs, OPOs are more efficient and can provide shorter pulses with increased wavelength tunability.
If successful, this new work will enable wider access to deeper views into tissue and provide insight into some of the world’s most debilitating diseases.
Meet the authors
Christopher Leburn, Ph.D., is co-founder and commercial director of Chromacity Ltd., where he is responsible for the commercial growth of the ultrafast laser manufacturer within academia. He holds a doctorate in laser physics from the University of St. Andrews and was awarded a Royal Society of Edinburgh Enterprise Fellowship in 2014; email: [email protected].
Christian Wilms, Ph.D., is the research and development manager at Scientifica Ltd., where he is responsible for helping to bring advanced technologies to a wide range of biomedical researchers. A neurobiologist by training, Wilms holds a doctorate from Leipzig University and has a keen interest in nonlinear biomedical imaging; email: [email protected].
/Buyers_Guide/Scientifica_Ltd/c13277
/Buyers_Guide/Chromacity_Ltd/c24437
Published: September 2021
FeaturesLasersImagingMicroscopyfiber lasersultrafast lasersTunable LasersBiophotonics