A recent clinical study has shown that indocyanine green dye, which provides near-infrared fluorescence, can highlight clotting in the vasculature that is not visible with other techniques.
SAMUEL S. STREETER, GABRIELLE S. RAY, JONATHAN THOMAS ELLIOTT, and ERIC R. HENDERSON, DARTMOUTH HEALTH and the NEFARIOUS STUDY GROUP
Necrotizing soft-tissue infections (NSTIs) are aggressive and rapidly advancing infections with a mortality rate between 20% and 30%1,2. In the mainstream media, this disease process is more commonly referred to as “necrotizing fasciitis” or “flesh-eating bacteria.” A recent clinical study has shown that a common fluorescence agent can pinpoint these NSTIs in their early stages — potentially improving chances for survival.
NSTIs occur when highly virulent bacteria are inoculated in or around human fascia, the connective tissue that surrounds and supports critical structures in our bodies. Fascia is a permissive environment for bacteria to multiply, leading to the rapid spread of infection. With little warning, often within hours, NSTIs can progress from mild skin changes to sepsis, multi-organ failure, and death. NSTIs primarily affect older and infirm patients. However, people of all ages and backgrounds may be affected by NSTIs, including children and neonates.
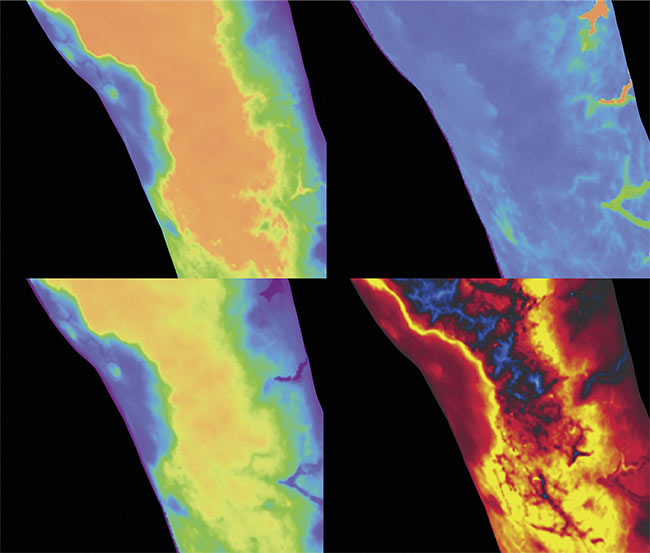
The panels in the image depict dynamic contrast-enhanced fluorescence imaging first-pass kinetic parameters derived from video-rate imaging of indocyanine green fluorescence, quantifying tissue perfusion in an infected limb. (Clockwise, from top left) Max intensity, time-to-peak, ingress slope, and egress slope. Courtesy of Dartmouth Health.
A major obstacle to prompt NSTI treatment is the lack of a definitive diagnostic test. Patients with NSTIs often suffer from nonspecific symptoms including pain, fever, and inflammation. Current diagnostic practices for these infections include clinical scoring systems, such as the frequently employed Laboratory Risk Index for Necrotizing Fasciitis (LRINEC) score, which attempts to differentiate patients from those stricken with other less emergent soft-tissue infections3. Additional accepted diagnostic methods include frozen section biopsy and radiological imaging, but none have a high degree of accuracy. The rate of initial misdiagnosis of NSTIs is 71% to 85% and is considered the most important predictor of mortality4,5. Emergent intravenous antibiotics and surgical debridement (i.e., the removal of tissue) have been the standard-of-care for decades.
Surgical treatment relies on clinical gestalt and visual and tactile inspection by the surgeon with the goal of thorough debridement of all infected tissues. Because NSTIs are so morbid, surgical debridement must be performed with the intent to remove all infected tissues without concern for the preservation of important structures, such as major blood vessels and nerves. NSTIs can lead to permanent loss of function and/or limb amputation. The current standard of care does not incorporate quantifiable criteria to guide debridement. So the rate of repeat debridement surgeries is high, amplifying undue suffering of patients. Given that these infections are relatively uncommon, NSTIs are very difficult to study due to a lack of sample data, limiting the ability of biomedical research to lead to improved treatments and outcomes. A major clinical need exists for rapid, accurate detection of NSTIs.
The role of indocyanine green
A key histological feature of NSTI-affected tissues that has so far not been fully exploited for diagnostic purposes is prominent blood vessel thrombosis (clotting). This clotting is a consequence of causative bacteria producing what is known as tissue factor, which is a protein that initiates the body’s coagulation cascade. While this clotting may be invisible to the naked eye, researchers at Dartmouth College and Dartmouth Health, led by Eric Henderson and Jonathan Thomas Elliott, have discovered that the systemic administration of indocyanine green (ICG), a near-infrared fluorescent agent, enables the visualization of superficial blood vessel thrombosis caused by NSTIs.
ICG has an excellent safety record and more than 60 years of Food and Drug Administration (FDA) approval for angiography, ophthalmology, and liver function assessment. ICG binds to albumin in the blood, making it a non-tissue-specific, vascular perfusion-based fluorescent agent. Within seconds of intravenous administration, ICG enables visual demarcation of local vasculature, distinguishing perfused tissues from non-perfused tissues (opening image). Dynamic-contrast enhanced fluorescence imaging (DCE-FI) using ICG involves collecting time-series fluorescence images over tens of seconds to minutes to measure first-pass perfusion kinetic parameters in target tissues (e.g., blood flow, blood volume, vessel permeability, and retention)6,7.
Results from a clinical pilot study at Dartmouth Hitchcock Medical Center in Lebanon, N.H., suggest ICG-based DCE-FI may be a valuable tool for rapid detection of NSTIs8. The study, funded by a Hitchcock Foundation pilot grant awarded to Gabrielle Ray, Henderson, and Elliott, is approved by the Dartmouth Institutional Review Board and involves imaging patients suspected of having NSTIs who arrive at the Dartmouth Hitchcock Medical Center Emergency Department. Eligible patients are informed about the study, and those who provide consent receive a single, weight-based dose of ICG (0.2 mg/kg), followed by up to 4 min of video-rate fluorescence imaging using a commercial fluorescence imager (SPY Elite or SPY Portable Handheld Imager system from Stryker).
Custom imaging system accessories enable quantitative comparisons in measured fluorescence between acquisitions and patients (Figure 1). The process takes <10 min per patient. The care team is blinded to the imaging results, and the imaging does not interfere with patient care. The primary finding thus far is that ICG-based DCE-FI is capable of revealing NSTI-caused vessel thrombi. These thrombi prevent the perfusion of ICG through infected tissues, resulting in prominent fluorescence signal voids in areas impacted by the necrotizing infection, while surrounding tissues fluoresce brightly. Representative cases from the pilot study are shown in Figure 2.
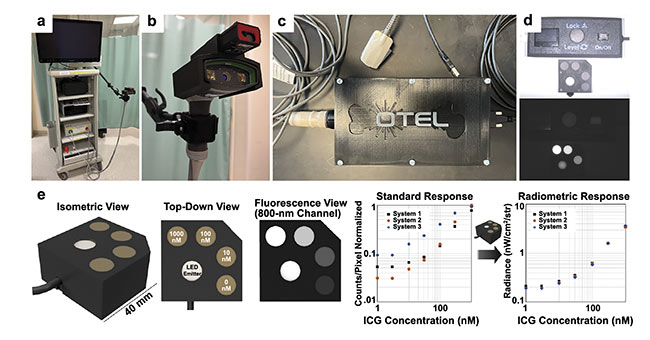
Figure 1. The imaging instrumentation for the pilot study includes a cart-based commercial infrared fluorescence imaging system (pictured here is a SPY Portable Handheld Imager by Stryker) (a); a custom SPY PHI attachment with range finder to maintain a constant working distance (b); a custom densitometer for measuring patient arterial input functions, which can be used to correct for differences in cardiac output and baseline vascular perfusion on a patient level (c); a custom indocyanine green (ICG)-equivalent fluorescence phantom pictured under room light (top) and using 800-nm fluorescence excitation (bottom, d); and a calibrated radiance source, which corrects for phantom material photobleaching and is used to normalize fluorescence measurements between acquisitions and imaging systems (e). Courtesy of QUEL Imaging.
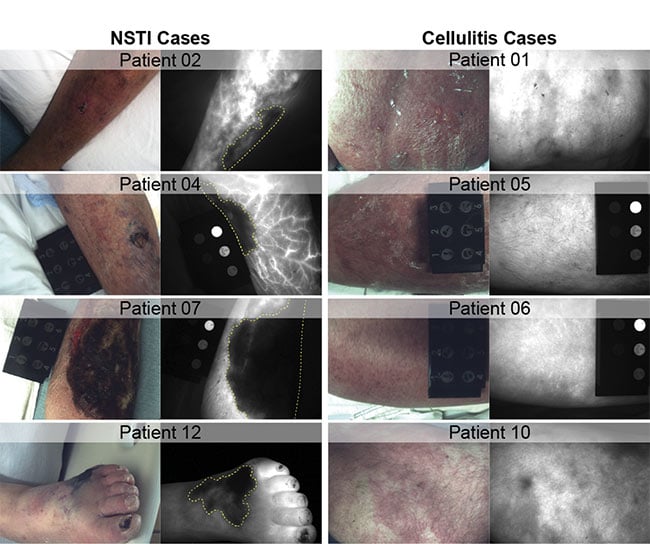
Figure 2. Representative room light and 800-nm channel indocyanine green (ICG) fluorescence images from the Dartmouth pilot study demonstrating confirmed necrotizing soft-tissue infections (NSTIs) (left column), and cellulitis infections (right column). Prominent signal voids are present in all NSTI cases (yellow dashed lines), indicative of NSTI-caused tissue breakdown and vascular thrombosis. Cellulitis cases exhibit marked ICG hyperintensity in affected tissues without signs of thrombosis. Courtesy of Dartmouth Health.
Effective differentiation
The pilot study to date has identified predominantly two groups of patients: those with true NSTIs and those with cellulitis, a common skin infection with a substantially better prognosis, requiring only antibiotic treatment (i.e., unlikely to need surgery, and presents a low risk of imminent, life-threatening complications). In clinical practice, cases of NSTIs are particularly difficult to differentiate from cases of cellulitis in the context of traditional clinical protocols. Early symptoms for these two types of soft-tissue infections are effectively indistinguishable, but importantly, cellulitis infections do not cause vessel thrombosis. The Dartmouth team has observed that ICG fluorescence imaging of cellulitis infections results in a “glow ball” imaging signature without any prominent signal voids, suggesting that the infection in question is not an NSTI. This fluorescence signature is realized after only 20 to 30 s of imaging.
The compelling pilot study results thus far are largely based on the analysis of single ICG fluorescence images or snapshots, but the Dartmouth research team sees much greater potential for video-rate ICG imaging (i.e., DCE-FI). ICG-based DCE-FI enables the quantitative measurement of tissue perfusion kinetic parameters, because frame-by-frame measurements of fluorescence intensity allow for temporal tracking of blood perfusion into and out of tissues. This is achieved using pixel-level tracking of fluorescence intensity with respect to time, which generates a characteristic curve with a rapid ingress slope, a peak intensity, and then a relatively gradual egress slope in intensity. Figure 3 illustrates the concept of DCE-FI perfusion kinetic parameterization in one confirmed NSTI-positive case.
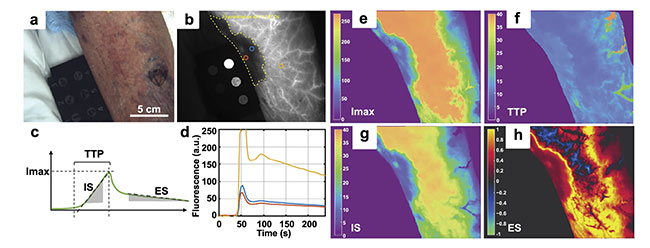
Figure 3. A representative case of confirmed necrotizing soft-tissue infection (NSTI) in the lower leg (Patient 04 from Figure 2); room light image with no contrast to disease extent (a); indocyanine green fluorescence reveals the approximate extent of the disease represented by a signal void (yellow dashed line, b); curve parameterization yields four features: max intensity (Imax), time-to-peak (TTP), inflow slope (IS), egress slope (ES) (c); kinetic curves from regions of interest (b-d). Parameter maps are shown for Imax (e), TTP (f), IS (g), and ES (h). Adapted with permission from Reference 5.
Single-frame contrast metrics and DCE-FI features extracted from pilot study data show a clear separation between NSTI and cellulitis cases, suggesting that the approach offers high diagnostic accuracy for distinguishing these infections (Figure 4). However, the pilot study data set is limited in case numbers and the diversity of condition (e.g., causative pathogens, infection location and depth below skin, and skin pigmentation).
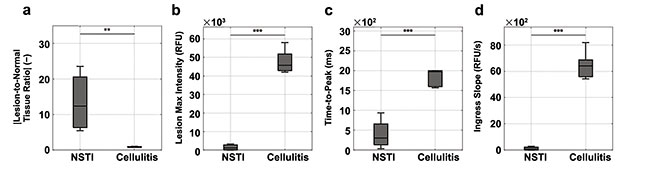
Figure 4. Indocyanine green (ICG) fluorescence metrics distinguish necrotizing soft-tissue infections (NSTIs) and non-NSTIs (i.e., cellulitis): signal-to-background (a) and dynamic contrast-enhanced fluorescence imaging first-pass kinetic curve parameters (b-d). RFU: relative fluorescence units. One-way analysis of variance results: *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001. Courtesy of Dartmouth Health.
Relatively few patients have been imaged thus far in the study; only 16 patients have been enrolled in the study to date. Additionally, it is well known by researchers and clinicians that necrotizing infections can spread in deep tissues, well beneath the 1- to 2-cm depth sensitivity afforded by near-infrared fluorescence imaging. The patients’ levels of skin pigmentation will also affect imaging results. Additional data collection is required to fully characterize the extent of these limitations. Future work led by the Dartmouth team will focus on both clinical and preclinical studies to evaluate ICG fluorescence imaging as a component of NSTI treatment. There is potential for two distinct applications of DCE-FI using ICG: first, for the detection of NSTIs versus other less deadly infections, which is the focus of the ongoing pilot study; and second, determining the extent of NSTI-affected tissues such that imaging may provide real-time guidance during surgical debridement (Figure 5). The latter application will be the focus of future studies.
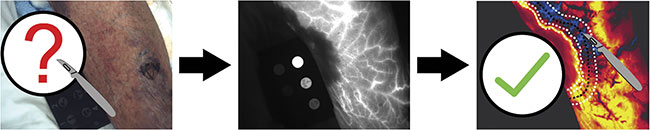
Figure 5. A conceptual rendering of how indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging may prove to be valuable for determining the spatial extent of necrotizing infection, thereby providing real-time guidance during surgical debridement of affected tissues. Courtesy of Dartmouth Health.
ICG research expands
For future clinical work, the Dartmouth team is actively collaborating with several medical centers across the country. Encouraged by promising preliminary results, Henderson and Elliott have formed the NEFARIOUS (Necrotizing Fasciitis Research in Orthopedics, Urology, and Surgery) study group, which includes physician-scientists from across the country with firsthand experience treating patients with NSTIs and with a vested interest in helping evaluate ICG fluorescence imaging for improving care for these patients. NSTI-causing pathogens vary geographically, primarily between inland and coastal sites, and NSTIs can be polymicrobial or monomicrobial in nature. The most common causative pathogen in both poly- and monomicrobial NSTIs is group A Streptococcus, but other common pathogens include Staphylococcus aureus, Vibrio vulnificus, Clostridium perfringens, and Aeromonas hydrophila. Collaborating centers will provide patient enrollments from sites across the U.S., so that DCE-FI using ICG can be evaluated on a wide range of NSTIs.
“We hypothesize that our ICG kinetic imaging will not be impacted by different types of causative bacteria, but we need to rigorously test this to find out,” said Elliott, co-principal investigator for the ongoing study. The research team is currently pursuing funding from the National Institutes of Health for a multicenter observational clinical study to expand the existing sample set.
For future preclinical work, the team plans to partner with leading experts in bacteriology to develop preclinical models of necrotizing infection. These models will enable a controlled, reproducible study of these infections in laboratory animals. Through these models, the team hopes to learn how ICG fluorescence imaging kinetic parameters relate to the underlying presence and extent of infections based on co-registered microscopic histopathology and quantitative polymerase chain reaction assays. The models will allow the research team to investigate whether fluorescence imaging with ICG identifies the boundaries of infection, and how the boundaries evolve over the course of NSTI advancement. This kind of disease modeling and pathological analysis would not be possible with human subjects but is essential for a complete understanding of the pathophysiological phenomena captured by the ICG imaging.
The ongoing and future research studies led by the Dartmouth research team have the potential to yield groundbreaking improvements in the treatment of NSTIs, particularly since ICG is already approved by the FDA for medical use, and it is already compatible with a number of commercial, FDA-approved fluorescence imaging systems. Successful completion of the current pilot study and upcoming multicenter study will lead to the pursuit of additional funding to explore ICG-based DCE-FI as a tool to detect and guide surgical debridement for NSTIs in a variety of medical settings. If results continue to be positive, the Dartmouth team hopes to ultimately advance ICG-based DCE-FI via the FDA Investigational New Drug pathway to pivotal trials in the coming years.
Meet the authors
Samuel Streeter is an assistant professor of orthopedics at the Geisel School of Medicine at Dartmouth and a scientist in the Department of Orthopedics at Dartmouth-Hitchcock Clinic. His current research interests and funded projects involve wide field-of-view optical imaging, fluorescence imaging, and image processing for surgical guidance; email: [email protected].
Gabrielle Ray is a resident physician training in orthopedic surgery at Dartmouth-Hitchcock Medical Center. Ray has clinical research experience in the implementation of clinical studies in the Department of Orthopedic Surgery at Massachusetts General Hospital, in Neurocritical Care at Maine Medical Center, and in the Department of Orthopedics at Dartmouth-Hitchcock Medical Center; email: [email protected].
Jonathan Thomas Elliott is an assistant professor of surgery at Geisel School of Medicine at Dartmouth and a senior scientist at Dartmouth-Hitchcock Clinic. He is an adjunct assistant professor of engineering at Thayer School of Engineering at Dartmouth. Elliott directs the Translational Engineering in Orthopedics Laboratory based in the Department of Orthopedics at Dartmouth-Hitchcock. He has developed optical imaging systems to improve detection and treatment of orthopedic trauma, infection, and cancer; email: [email protected].
Eric Henderson is an orthopedic surgeon specializing in bone and soft-tissue tumors of the extremities and pelvis. Henderson’s research involves improving the safety and efficacy of surgery for patients requiring complex limb salvage operations for cancer and infection through advanced optical navigation. Henderson is an adjunct associate professor at the Thayer School of Engineering at Dartmouth; email: [email protected].
References
1. D.L. Stevens et al. (2017). Necrotizing Soft-Tissue Infections. N Engl J Med, Vol. 377, No. 23, pp. 2253-2265.
2. F. Nawijn et al. (2020). Time is of the essence when treating necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg, Vol. 15, p. 4.
3. C-H Wong et al. (2004). The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med, Vol. 32, No. 7, pp.1535-1541.
4. C-H Wong et al. (2003). Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am, Vol. 85, No. 8, pp.1454-1460.
5. T. Goh et al. (2014). Early diagnosis of necrotizing fasciitis. Br J Surg, Vol. 101, No. 1, pp. e119-e125.
6. J.T. Elliott et al. (2020). Intraoperative fluorescence perfusion assessment should be corrected by a measured subject-specific arterial input function. J Biomed Opt,
Vol. 25, No. 6, pp. 1-14.
7. I.L. Gitajn et al. (2020). Evaluation of bone perfusion during open orthopedic surgery using quantitative dynamic contrast-enhanced fluorescence imaging. Biomed Opt Express, Vol. 11, No. 11, pp. 6458-6469.
8. S.S. Streeter et al. (2023). Early identification of life-threatening soft-tissue infection using dynamic fluorescence imaging:
first-in-kind clinical study of first-pass kinetics. Proc SPIE Int Soc Opt Eng,
Vol. 12361:123610B.