JOHANNESBURG, Sept. 4, 2023 — Photocatalysts show the potential to be used in energy generation and environmental detoxification applications. This is an intriguing quality, since reducing energy use in bulk industrial processes can create a trade-off between efficiency and environmental harm. Fossil fuels, for example, have helped humanity expand and innovate for hundreds of years. But their environmental toll and inevitable depletion create a need for a safer and more abundant resource, like solar power.
Highly effective photocatalysts, however, tend to be very expensive, as well as difficult and dangerous to develop. Many contain precious metals such as platinum and gold, which also have a negative environmental impact.
Researchers at the University of Johannesburg have developed a photocatalyst that can harness the visible portion of light given by the sun. This differentiates the photocatalyst from others, like titanium dioxide, which can capture only the ultraviolet (UV) spectrum. The advancement points to the newly developed photocatalyst’s utility for multiple industrial applications where sunlight or electrical light is available to facilitate chemical processes. Notably, the researchers said, the photocatalyst could be used for bulk water treatment. The researchers are currently testing the photocatalyst’s ability to break down organic pollutants and pharmaceutical residues in actual wastewater samples at laboratory scale.
The Johannesburg team’s photocatalyst is almost 90% off-the-shelf ingredients — around 89% graphitic carbon — and 10% calixarene, which catches and breaks down unwanted organic molecules. The photocatalyst can be produced at scale in resource-constrained laboratories. An additional ingredient is niobium carbide MXene. This nanomaterial is used in photocatalytic applications such as hydrogen generation and carbon dioxide conversion.

University of Johannesburg photocatalyst research team: From left, project supervisor Langelihle (Nsika) Dlamini, project collaborator Edwin Mmutlane, and study first author L. Collen Makola. Courtesy of University of Johannesburg.
According to study first author L. Collen Makola, the MXene prevents photo-generated electrons from the graphitic carbon nitride to recombine, or “cancel out,” with the positively charged species under visible light exposure. Both the calixarene and the MXene contributed to shifting of the light absorption into the visible region of the solar spectrum, Makola said.
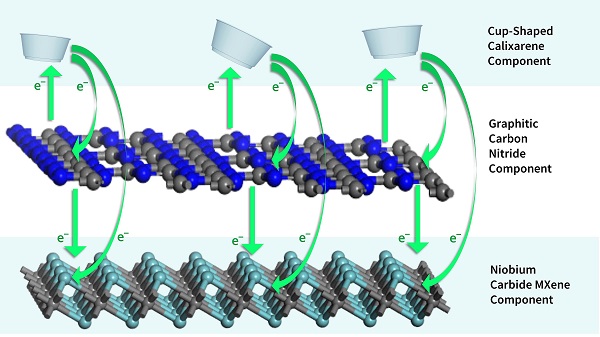
Calixarene, a graphite carbon nitride compound, and MXene come together to create an eco-friendly photocatalyst. Courtesy of University of Johannesburg.
This factor allows the photocatalyst to capture a significant amount light from the sun — about a third of the visual spectrum alongside the UV spectrum. That makes up around 18% of all available solar energy compared to the photocatalysts that are in use today, which can harness only the 5% that makes up UV. The researchers’ photocatalyst covers all wavelengths from 200 to 700 nm, which includes the violet-blue-cyan-green portion of the visible band.
Photocatalysts, Makola said, can be measured by evaluating their ability to convert solar and/or light energy into chemical energy. This is called photo-to-chemical conversation efficiency and represented as “mu.”
“Our photocatalyst (mu = 4.86%) surpasses a three-component photocatalyst (mu = 1.81%), which appeared in a journal article in 2017. That photocatalyst was composed of silver, cadmium sulfide, and zinc oxide,” Makola said.
The work was published in Journal of Science: Advanced Materials and Devices (www.doi.org/10.1016/j.jsamd.2023.100593).